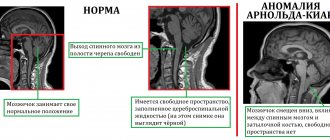
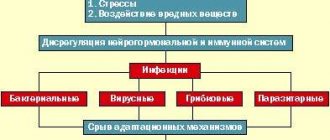
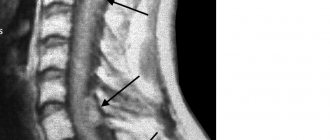
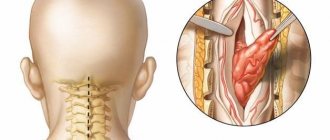
The problem of treating patients who have suffered a spinal cord injury is one of the most difficult in the neurorehabilitation system. Such patients are psychologically and/or economically dependent on their loved ones and on society, since their movements are often significantly limited and the natural functioning of vital body systems is disrupted [1, 2].
Numerous modern studies have demonstrated great promise for the use of electrical stimulation of the spinal cord in motor rehabilitation of patients with long-term consequences of spinal cord injury. It has been proven that several years after the injury, after complete paralysis, patients can stand and walk independently after a course of rehabilitation using epidural electrical stimulation of the spinal cord [3-5]. It has been proven that the mechanism of action of epidural and transcutaneous electrical stimulation of the spinal cord (TESCS) is the same [6]: TESC, as well as epidural electrical stimulation, can be successfully used to restore the motor functions of patients paralyzed after a spinal cord injury due to its complete motor damage [7 -9].
A feature of most previous works that showed the capabilities of electrical stimulation of the spinal cord in restoring voluntary movements and independent maintenance of a vertical posture is that regular, often daily, stimulation effects and accompanying motor training lasted 5-9 months and all motor training was carried out by the efforts of 2- 3 methodologists in the presence of a physiologist or doctor [4, 5, 10]. That is, impressive results were achieved over a long period of time with the involvement of a large number of specialists. In fact, the vast majority of work in this area are expensive scientific studies conducted under specific experimental conditions on patients selected in accordance with the requirements of a particular study. It remains unclear how effective a course of electrical stimulation of the spinal cord is in restoring the motor activity of patients who have suffered a spinal cord injury outside of the limitations and experimental conditions.
Spinal cord injury is accompanied not only by motor impairments. It is important to evaluate the effectiveness and safety of electrical spinal cord stimulation procedures primarily for restoring bladder, bowel and sexual function in patients with spinal cord injury, since the greatest suffering in spinal patients is caused by violations of these functions [11]. Spinal cord stimulation with parameters used to restore motor function has previously been shown to affect urinary function [12, 13]. Recently published results of a urodynamic study showed that in patients with spinal cord injury who do not control bladder function, a single tESCS at the level of the Th11-12 vertebrae reduces detrusor overactivity, regulates detrusor-sphincter mismatch, increases bladder filling and facilitates urination [14]. Thus, it can be expected that a short-term course of electrical stimulation of the spinal cord, aimed at restoring motor activity, can have a positive effect on the rehabilitation of excretory functions.
The purpose of the study is to determine the effectiveness of using tESCS in combination with standard rehabilitation of patients who have suffered a spinal cord injury. An additional goal is to evaluate the effect of tESCS on excretory functions in patients with spinal cord injury.
Material and methods
The studies were carried out on the basis of St. Petersburg State Budgetary Healthcare Institution “City Hospital No. 40”. The purpose, objectives and protocol of the study were approved by the scientific problem committee (protocol No. 142). Informed written consent to participate in the study was obtained from patients.
Inclusion criteria for the study: age over 18 years, mid- and lower-thoracic level of injury, duration of injury more than 9 months, severity of spinal cord injury according to the American Spinal Injury Association (ASIA) scale - B or C, muscle tone of the lower extremities on the modified Ashworth scale - 2 or 3 points.
Exclusion criteria: severe concomitant diseases in the stage of decompensation, damage to the skin in the area where the electrodes are located, the presence of instability in the elements of the musculoskeletal system, mental illness.
The study involved 15 patients with late-stage spinal cord injury (Table 1;
Table 1. Description of study participants. Changes in motor activity indicators before and after treatment Note. * — patients underwent rehabilitation on an outpatient basis. Female patients are in italics, differences in indicators before and after the course of treatment are in background, positive dynamics are in bold, and negative dynamics are in regular font. patients are ranked within each group according to the duration of the period after injury). All patients underwent a standard course of rehabilitation treatment. The rehabilitation course consisted of physical therapy classes, mechanotherapy, including robotic therapy (Lokomat, Hocoma, Sweden), massage, and physiotherapy, performed daily, 10 procedures per course. The duration of the study was ~2 weeks.
The main group (Nos. 1-7 in Table 1) consisted of patients who agreed to undergo the TESCM procedure. They received a standard course of therapy and TESCM procedures. Patients in the control group (nos. 8-15 in Table 1) received only standard treatment.
The BioStim-5 device (Kosima LLC) was used for ESSM. Electrodes (WFB02 QWER, China; BF4, LEAD-LOC, Inc., USA) with an adhesive conductive layer were fixed cutaneously. A stimulating electrode (cathode) in the form of a disk with a diameter of 2.5 cm was placed on the midline of the spine between the spinous processes at 3 levels: C5-6, Th11-12 and L1-2. Indifferent electrodes (anodes) were placed symmetrically over the iliac crests. Stimulation was carried out by rectangular pulses (monopolar and/or bipolar) with a duration of 1 ms, filled with a carrier frequency of 10 kHz. The magnitude of the current was selected individually so that it was not painful and at the same time caused contraction of the muscles of the lower extremities. The amplitude range of the currents used was 30–120 mA. During the procedure, the current value was gradually increased by 20-40 mA. The pulse frequency was 15–30 Hz. TESSM was carried out simultaneously with motor training on a simulator for active-passive rehabilitation of the upper and lower extremities (Thera vital, “Medica Medizintechnik”, Germany). The training regimen was selected individually depending on the functional abilities of the patients and the assigned rehabilitation tasks. Also, to improve statics, exercises were used while sitting on a chair, standing in a knee support with a gradual decrease in the area of support. When choosing the parameters of stimulating effects, we were guided by the results of using tESCS to regulate locomotor [8] and postural functions [10] in patients with vertebrospinal pathology.
The duration of the complex procedure was 30 minutes; there were 10 procedures per course, performed daily, 5 times a week.
Standard scales were used to assess the neurological status of patients before and after the course of treatment. To determine the severity of spinal injury in terms of sensitivity and muscle strength, the ASIA/ISNCSCI scale (International Standards for Neurological and Functional Classification of Spinal Cord Injury) was used. The Ashworth scale was used to determine muscle spasticity. The strength of the flexor and extensor muscles of the hips, legs and feet was assessed using the 6-point British Medical Research Council scale (Harrison scale) [15]. Also, the standard neurological examination included determining the level of hypo- and anesthesia, the presence of deep sensitivity and muscle-articular sensation. Patients filled out a urination diary, in which they noted the urge to urinate and its control. The amount of residual urine was monitored using bladder catheterization or ultrasound. All studies were carried out before the start of the rehabilitation course and immediately after its completion.
Statistical analysis of changes in muscle strength and sensitivity was performed using Student's t test and the nonparametric Wilcoxon test, comparing values for both legs of the patient without averaging them. Differences between indicators were considered statistically significant at p
<0.05 for each criterion. Other indicators were compared using the Mann-Whitney or Wilcoxon tests.
results
The average age of patients in the control group was 27.9±3.80 years, in the main group - 29.9±8.07 years. The average period after injury was 2.8±2.21 and 1.9±1.06 years in the control and main groups, respectively. There are no statistically significant differences between the groups in these parameters. Spasticity scores in the control and main groups were 2.7±0.39 and 2.1±0.35 points on the Ashworth scale, respectively. Initially, according to the scores of muscle strength, sensitivity, and recorded indicators of the urinary system, the groups were homogeneous among themselves and did not have statistically significant differences (see Tables 1 and 2).
Table 2. Changes in sensitivity and excretory function before and after treatment Note. * — 0 — no control, 1 — partial control, 2 — full control; ** - 0 - >100 ml, 1 - 50-100 ml, 2 - <50 ml. The differences in indicators before and after the course of treatment are highlighted in background, positive dynamics are in bold, and female patients are in italics.
During the rehabilitation treatment, no adverse events (illnesses, injuries, unplanned surgical interventions, etc.) occurred. There was an increase in spasticity by 1 point at the end of the course in 1 patient from the main group (O8) and 2 patients from the control group (K5, K9).
According to the results of the study of muscle strength, all patients of the main group, in whom muscle strength in the lower extremities was recorded at the beginning of the course, noted its improvement to 2-3 points (patients O5, O8, O10, O12), in 2 more patients who had previously had no muscle activity in the lower extremities, muscle strength appeared up to 2 points (patients O2, O4). Subjectively, patients noted that during the TESCM procedure it was easier for them to exercise on the simulator, stand or perform other doctor’s tasks, they began to feel tension and work of the muscles of the lower extremities, their regulation improved, which significantly influenced the emotional mood of the patients and further motivation to exercise.
In the control group, 1 patient whose muscle strength was recorded at the beginning of the course also showed an increase to 2 points (patient K10).
Comparison of muscle strength scores recorded before and after the course using statistical criteria revealed in the main group a significant increase in the strength of the flexor and extensor muscles of the hip and extensor muscles of the leg ( p
<0.05 by both Wilcoxon and Student's tests).
Statistical analysis of changes in muscle strength in the control group showed a significant increase in the strength of the hip flexor and extensor muscles according to the Wilcoxon test ( p
<0.01) and non-significant according to the Student test (
p
= 0.838). Due to the striking differences between the probabilities calculated using the 2 criteria, no conclusion was made about the statistical significance of the changes in muscle strength in the control group.
When studying sensitivity (see Table 2), in 1 patient of the main group the level of anesthesia dropped by 1 segment. Another 2 patients developed deep sensitivity and muscle-articular sensation in the knee joints (patients O2, O10). Comparison of sensitivity scores recorded before and after the course using statistical tests did not reveal significant differences.
In the control group, no change in sensitivity was noted after the course.
The increase on the ASIA scale in the main group ranged from 1 to 8 points. Patients O2 and O4 were reclassified the level and severity of injury on this scale from B to C (see Table 1).
The increase on the ASIA scale in the control group did not exceed 1 point.
After analyzing the urination diaries kept by the patients, it was revealed that in the main group, 1 out of 3 patients who did not control urination developed the urge to urinate, and he also regained partial control of urination (patient O2). Another 1 patient (O3) also developed partial urinary control. In both cases, the effect of TESCM on urinary function was noted after 3-5 procedures. At the beginning of the course, in 4 patients of the main group (O2, O3, O5, O8), the amount of residual urine was more than 100 ml. At the end of the TESCM course, 2 patients (O3 and O8) had from 50 to 100 ml of urine left after urination, and 1 patient (O2) had less than 50 ml, which corresponds to normal values and allowed him to catheterize less often. In the group as a whole, the differences in the analyzed indicators of urinary function were statistically insignificant.
In the control group, the analyzed indicators of urinary function remained unchanged after the course of treatment.
Basic studies of pain using the VAS scale
All patients with a positive test period noted the appearance of a slight tingling sensation and/or warm waves in the part of the body where they usually felt pain. These sensations were comfortable for patients. Immediately after surgery in this group of patients, VAS scores were no higher than 5 points, and satisfaction levels were also in the range of 50-90%. The follow-up period in our group of patients ranged from 1 to 26 months.
Six patients (9.7% of the total number of patients and 11.5% of patients with a positive test period) began to notice a decrease in the analgesic effect from the stimulation performed within 15-21 months after surgery. We regarded this phenomenon as a “stimulation habituation effect.” Thus, in our series of 62 patients, a stable effect from analgesic stimulation was achieved in 46 patients (74.2%). Perhaps, with an increase in the period of observation of operated patients, we will see a decrease in the number of good results.
Thus, the selection of patients for the use of this technique must be done with extreme care in strict accordance with the following criteria:
- disabling nature of the pain syndrome;
- absence of severe psychological disorders in the patient;
- the patient must adequately assess his condition and the possibilities of upcoming surgical treatment;
- the presence of a good analgesic effect in the test period;
- The patient during the operation and in the postoperative period must be able to correctly follow the doctor’s instructions and periodically undergo follow-up examinations.
This method is not a panacea for all ills, and its clinical effectiveness, according to large-scale and long-term studies, does not exceed 55-70%, i.e. the potential failure rate is quite high. In view of this, careful selection of patients is, although not absolute, insurance against negative results and disappointment for both the patient and the surgeon.
Related links:
- Spinal canal stenosis
- The effectiveness of epidural electrical stimulation in myelopathy of various origins
- Experience with chronic epidural neurostimulation
Also worth reading:
Discussion
The main result of the study is evidence of the effectiveness of the use of tESCM in motor neurorehabilitation. As a result of the course of treatment, a number of patients showed improvement in motor and excretory functions. Thus, after a 2-week course of TESCM in combination with motor therapy and a standard course of restorative treatment, consisting of massage, physical therapy, and mechanical therapy, 6 out of 7 patients showed an increase in muscle strength of the lower extremities, and 3 had an improvement in sensitivity indicators. After completion of the course, in 2 patients the severity of the injury was reduced from ASIA B to ASIA C.
A previous study using tESCS during a short course of motor rehabilitation was aimed at studying the combined effects of spinal cord stimulation and activation of serotonin receptors, so it did not include a group of patients who did not receive stimulation [16]. The group of participants included both patients with spinal cord injury and patients with iatrogenic myelopathy; the course consisted of 16-17 half-hour TESCM procedures against the background of mechanotherapy; half of the patients received buspirone, a serotonin receptor agonist. As a result of the course, on average for the group, a significant increase in muscle strength and sensitivity was obtained. However, it is impossible to associate the results achieved after the rehabilitation course only with tESCM due to the lack of a control group of patients who did not undergo tESCM.
Thus, for the first time in a controlled study, we have shown that tESCS is effective in a short-term course of rehabilitation of motor functions in patients with ASIA B and C severity of spinal cord injury. If we look at studies in which it was demonstrated that a multi-month course of electrical stimulation of the spinal cord leads to restoration of independent walking in patients with severe motor impairments [3-5], it will become obvious that the long process of walking restoration consisted of short courses changing one into another, during which certain rehabilitation tasks were solved. The patients were consistently restored: voluntary movements of the legs in conditions of their external support (when gravity was compensated), the ability to maintain a vertical posture, and independent regulation of stepping movements for the right and left legs separately. This gives reason to believe that 2-3-week courses of tESCM can be used in the conditions of inpatient or outpatient rehabilitation of spinal patients for multi-stage restoration of severe motor disorders, complicating the restored motor skills at each subsequent stage.
In the main group, 1 patient had an increase in spasticity of the lower extremities by 1 point after a course of TESCM. It should be noted that the study cited above [16] also recorded an increase in spasticity by 0.5 points after a course of TESCM. In our study, also in the control group, an increase in spasticity was observed at the end of the course in 2 patients. It seems unlikely that this is due specifically to spinal cord stimulation. Electrical epidural stimulation of the spinal cord is known to be used in some cases to reduce spasticity. In particular, a study was conducted on the possibility of using electrical stimulation of the spinal cord to reduce spasticity in patients with cerebral palsy and spinal cord diseases and injuries [17]. It was shown that stimulation at the Th11 level with a frequency of 100-130 Hz, pulse duration 120-300 ms, amplitude 1.5-4 V over several years led to a decrease in spasticity in both groups. In spinal patients, spasticity decreased from 3.71 ± 0.61 points before stimulator implantation to 2.26 ± 0.56 points after 1–9 years of stimulation. However, the authors of a recently published review [18] of a large number of publications that described cases of reduction in spasticity of the lower extremities due to electrical stimulation of the spinal cord, doubt the validity of the conclusions of these studies due to shortcomings in the methodology of the studies, due to technological limitations of implanted equipment and the lack of full understanding of the mechanisms of spasticity. Thus, the effect of tESCS on spasticity remains to be investigated.
Spinal injuries in a large number of cases cause disturbances in voluntary urination [19, 20]. Normally, the functioning of the bladder is associated with spinal cord segments T11-L2 and S2-S4 [21]. To rehabilitate motor functions of the lower extremities, as a rule, electrical stimulation of the spinal cord is performed at the T12-L4 level [4-6]. In this regard, it is logical to expect that by stimulating the spinal cord to restore motor functions, it is possible to influence excretory functions. In spinal animal studies, electrical stimulation of the spinal cord with parameters used for motor rehabilitation has been shown to affect micturition function [12, 13]. A urodynamic study was performed on spinal patients during the TESCM procedure at the T11 vertebral level [14]. This study found that 30 Hz stimulation reduced detrusor overactivity, detrusor and sphincter dyssynergia, and 1 Hz stimulation initiated bladder emptying. In our study, it was found that a course of tESCS with similar stimulation parameters leads to partial normalization of urinary functions in 3 out of 7 patients. Thus, motor rehabilitation using tESCM may be accompanied by an improvement in excretory functions, and it is obvious that further research is required in order to use tESCM to regulate voluntary urination after spinal cord injury.
Modern possibilities of neurostimulation
Kostetskaya Ksenia Andreevna specialist of the Neuro department, IMPLANTA company (St. Jude Medical)
In the practice of many spinal surgeons, it is not uncommon for a patient to undergo repeated surgical interventions: microdiscectomy, radiculolysis, facetectomy, but the pain, persistent and persistent, continues to torment him, worsening the quality of life, making the person feel helpless in society.
Despite the rapid development of modern surgical equipment, according to statistics, neurogenic pain after surgery develops in 20–40% of cases. This syndrome is called “Failed Back Surgery Syndrome” or FBSS. If you rely on the English name of the syndrome, you might think that these pains are the result of an unsuccessful operation. This is not at all true: operations, as a rule, are carried out brilliantly: the surgeon manages to stabilize the vertebrae or perform a masterly removal of a hernia, but pain, neurogenic pain, shooting, burning, begins to develop, despite the absence of a physiological cause.
The International Association for the Study of Pain defines FBSS as: “Lumbar (or cervical) pain of unknown origin or persistent pain that persists despite surgery or occurs after surgery in the same anatomical region.”
Patients who develop Operated Spine Syndrome usually consult a neurologist, undergo a course of pharmacotherapy (non-steroidal anti-inflammatory drugs, muscle relaxants, narcotic analgesics, antidepressants) and/or use physiotherapeutic devices, which often has a positive effect. But over time, drug-resistant forms of pain syndrome may develop. According to American statistics, there are up to 50 thousand such cases annually.
At this stage of treatment, the patient has no other options for pain control other than surgery. One way to eliminate neuralgia is to interrupt the transmission of pain signals. For example, perform a neurotomy procedure - it is done on nerve tissue to prevent the transmission of a pain signal. But this is risky, and most importantly, this procedure is irreversible.
As a result, after long-term unsuccessful conservative and surgical treatment, after 3-6 months a patient with FBSS may become disabled for life!
The only reversible method for managing chronic pain today is neuromodulation (or neurostimulation). The principle of action is the same: the pain signal going to the brain is stopped. But! There are no irreversible changes in the body associated with the operation. The doctor (and then the patient himself), using an implanted device, “closes the door” to pain: the patient stops feeling pain due to the fact that the signal cannot pass through. Since we are eliminating pain, and not masking the inflammatory process, the result is achieved: no side effects (no chemical changes), everything is reversible (all cells of the body, all neurons are intact, you can turn off the stimulation - and everything will return; therefore, if after 15 , in 20 years a new technology will be created, the patient will be able to try it, since there are no irreversible processes).
How stimulation works
Pain is a signal that goes from the anatomical zone through the spinal cord to the brain, which perceives it as pain. To interrupt this signal before it reaches the brain, an additional electrical impulse can be sent directly to the spinal cord at the exact point where the nerve endings of the diseased organ enter it (the pain signal will not be able to travel, and the brain will not receive the signal to pain). The operating principle is based on the theory of Ronald Melzack (Great Britain) and Patrick Wall (Canada), which was published in 1965 in the American journal Science. And two years later, based on this theory, American surgeon Norm Shealy performs spinal cord stimulation surgery to control pain in a patient suffering from cancer. Soon, spinal cord stimulators began to be actively used in pain therapy. Currently, about 100,000 systems are implanted annually in the world, and by the next decade (according to experts), this figure will increase to 240,000, due to the increase in patients with chronic diseases and the expansion of the geographical application of this therapy.
Certified for the management of chronic back, neck, arm and leg pain, neurostimulation is a proven therapy that has been used by physicians around the world for over 40 years to improve patient quality of life. In Russia, neurostimulation is included in the quota for neurosurgical high-tech treatment.
The first SCS (spinal cord stimulation) operation in the USSR was performed in 1987 at the Research Institute of Neurosurgery named after. N. N. Burdenko neurosurgeon, professor V. A. Shabalov. Since 1991, domestic models “Neuroelect” began to be used in Russia (developed by V. A. Shabalov together with the All-Russian Research Institute of Optical Physical Measurements). Unfortunately, the materials and quality of the models did not provide a stable long-term effect. Since 1995, American implantable systems have become available. But still, these were only a few: only 5–10 pieces per year. And only since 2001, with funding from the Ministry of Health and the Academy of Sciences of the Russian Federation, the number of such operations and clinics (in which they were carried out) has increased sharply: from 1 clinic in 2001 to 18 by now. The number of implanted SCS systems in Russia exceeded 1000 by 2013. Now spinal cord stimulation is carried out using modern high-tech devices everywhere. However, neurostimulation is not a cure for the cause of pain or a cure for the disease. It is a therapy that is designed to mask pain (block pain signals before they reach the brain). Neurostimulation is mainly used to treat FBSS or post-laminectomy syndrome and other neuropathies.
Neurostimulation relieves only neuropathic pain; other types of pain (such as dental, intestinal, periodic, acute injuries, etc.) will remain so that the body can properly cope with external and internal factors.
Rice. 1.Patient remote control (St. Jude Medical Inc.) - a device with which the patient regulates stimulation within the limits specified by the doctor
Rice. 2. Pulse generator. Implanted into a subcutaneous pocket. The smallest stimulator (Eonmini St. Jude Medical Inc.) is only 10 mm thick
Rice. 3. Electrodes for implantation into the epidural space (St Jude Medical Inc.)
The operation to implant the system is usually carried out in two stages:
1. At the test stage, it allows the doctor, together with the patient, to evaluate the result of therapy and understand whether this method helps him. Test implantation is an operation under local anesthesia, when a neurosurgeon, spinal surgeon or anesthesiologist installs a special test electrode through a Tuohy needle at the appropriate spinal level, guided by a somatotopic map. In operated spine syndrome, as a rule, this is the Th11-L1 level. Next, the electrode is connected to an external device, and a neurologist or clinical engineer from the manufacturer begins stimulation, checking that the stimulation (paresthesia) covers the entire pain area. If not, then under X-ray control the surgeon corrects the location of the electrode contacts. The test period can last from 2–3 to 7 days. At this time, the patient and doctor decide whether to install a permanent system;
2. Implantation of a permanent system, when the surgeon chooses which electrode is best for the patient. This depends on the nature of the pain: if it is more complex, you can install an electrode with a large number of contacts; if pain is more localized, you can again implant a cylindrical electrode along the needle, then tunnel it to the pulse generator. Modern models of generators are completely invisible under clothing. For example, the smallest stimulator is only 10 mm thick (Eonmini™ from St. Jude Medical Inc.).
Rice. 4. Doctor’s programmer – a computer on which the doctor sets all stimulation parameters: frequency, pulse width, step size and therapeutic window of stimulation intensity
The main benefits of neuromodulation include:
- neurostimulation is a minimally invasive modern method of treating chronic back pain;
- absence of destruction of nervous tissue;
- complete reversibility of the effect;
- treatment correction is non-invasive (invasive correction may include changing the position of the electrode);
- efficiency-cost ratio. The generator can operate for 10 years or longer (rechargeable models); the patient significantly reduces or completely stops taking medications; the number of days in hospital is reduced.
- and most importantly, the quality of life improves!