Definition
“Tethered spinal cord syndrome” is an adaptation of the English term “the tethered spinal cord syndrome” (Yamada S., 1981). “tether” in the original name , which literally means a leash for pets , is associated with the desire to most accurately reflect the essence of the pathological process it describes. It is the stretching of the spinal cord, which develops as a result of restriction of its mobility, that is, fixation, that leads to the negative impact and development of clinical manifestations of FSM.
Yamada, S. (Ed.). (2010). 3 Pathophysiology of Tethered Cord Syndrome. Tethered Cord Syndrome in Children and Adults. doi:10.1055/b-0034-80501
“tethered spinal cord” first used by Hoffman to describe the results of treatment of 31 patients suffering from pelvic disorders in combination with motor disorders and decreased sensitivity in the lower extremities. Symptoms in all patients regressed after excision of the thickened filum terminale. Based on the data obtained, the authors concluded that the pathological process develops as a result of stretching of the caudal parts of the spinal cord (Hoffman HJ, 1976).
Pathogenesis
It is known that the spinal cord is protected from excessive stretching by the so-called “dentate ligaments” , which fix the spinal cord in the lumen of the spinal canal (Tunituri AR, 1977). While at the level of the cervical and thoracic spine they are significantly developed (Breig A., 1970; Tubbs RS, 2001), at the level of the lumbar spine they are not , and stretching of the caudal parts of the spinal cord is prevented only by the elastic properties of the terminal filament (Tani S., 1991 ).
Yamada, S. (Ed.). (2010). 3 Pathophysiology of Tethered Cord Syndrome. Tethered Cord Syndrome in Children and Adults. doi:10.1055/b-0034-80501
When the terminal filament was stretched in patients with FSM, its elongation was observed by no more than 10%, while normally it elongates by 50% or more (De Vloo P., 2016). Light microscopy of a section of a pathologically altered filament terminale in patients with FSM revealed a predominance of fibrous tissue; when stained for elastin, its content was minimal (Hendson G., 2016). Based on the data obtained, it was hypothesized that SFSM develops as a result of tension in the caudal parts of the spinal cord between the last pair of odontoid ligaments and any inelastic structure that fixes it caudally (Yamada S., 1981).
Yamada, S. (Ed.). (2010). 3 Pathophysiology of Tethered Cord Syndrome. Tethered Cord Syndrome in Children and Adults. doi:10.1055/b-0034-80501
In 1981, S. Yamada, in an experiment on cats, demonstrated a slowdown in metabolism in the tissues of the caudal parts of the spinal cord during stretching and its subsequent restoration when the tension was removed. A few years later, in 1987, it was officially proposed that “tethered spinal cord syndrome” be considered a scientifically based clinical diagnosis (McLone D., 1987), from which point this term began to appear more and more often in the literature.
Currently, most domestic and foreign specialists characterize FMS as a combination of motor, sensory, trophic disorders in the lower extremities, as well as pelvic disorders and musculoskeletal deformities that develop as a result of immobilization and stretching of the caudal parts of the spinal cord in pathologies of the filum terminale (“tight filum”) terminale” – inelastic filum terminale, filum terminale lipoma, etc.), myelodysplasias (MMC, spinal lipomas), complex spinal dysraphisms (diastematomyelia, dermoid, epidermoid, enteric cysts, dermal sinus), as well as post-inflammatory and postoperative scar-proliferative changes terminal cistern (leptopachine meningitis, arachnoid cysts, etc.) (McLone DG, 1997; Iskandar BJ, 1998; Tortori–Donati P., 2000;).
The reasons for the development of FSM are most often the consequences of correction of spina bifida, as well as Spina Bifida Occulta
Classification
It is obvious that a significant contribution to the development of clinical symptoms in spinal dysraphism , in addition to stretching, can be made by volumetric effects (compression), deformation, as well as congenital disorders of cyto- and angiomyeloarchitecture (Kumar R., 2010). Based on the concept of FSM as a reversible pathological condition, S. Yamada and DJ Won proposed to separate processes caused by limited mobility of the spinal cord (true FSM) and conditions that have clinical manifestations similar to FSM, which include in their pathogenesis, in addition to stretching, other unfavorable factors effects (compression, deformation, etc.), as well as pathologies that are not at all related to SFSM, caused mainly by disruption of the formation of the spinal cord and having an unfavorable prognosis for surgery aimed at eliminating the fixation of the spinal cord (Yamada S., 2007). The first category included patients with anomalies of the filum terminale , hernias of the sacral localization and small caudal lipomas, in which the main contribution to the pathogenesis of clinical manifestations is made by longitudinal stretch of the spinal cord. The second category includes patients with myelomeningocele of the lumbar spine, lipomyelocele and repeated scar fixation of the spinal cord. In these patients, obviously, only part of the symptoms presented relates to FMS and can regress after surgery. The third category of patients included children with spina bifida of the thoracic spine, accompanied by congenital paraplegia and incontinence (Yamada, 2007).
Classification of SFSM according to Yamada, Won 2007
In accordance with the described categories, expected results and indications for surgery were distributed. When fixation was eliminated in the first category of patients, a complete regression of symptoms , and patients from the third category have practically no chance of restoring lost functions (Liptak GS, 1995).
Spinal cord reflexes
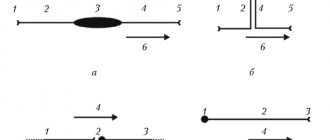
The operating principle of the segmental apparatus of the spinal cord is reflex arcs.
Basic diagram of the spinal cord reflex arc: information from the receptor
goes along
the sensitive neuron
, which switches to
the interneuron
, which, in turn, to
the motor neuron
, which carries information to
the effector organ
. The reflex arc is characterized by sensory input, involuntary, intersegmental, motor output.
Examples of spinal reflexes include:
- The flexor
reflex is a protective reflex aimed at removing a damaging stimulus (pulling the hand away from a hot one). - Stretch reflex
(proprioceptive) - prevents excessive stretching of the muscle. The peculiarity of this reflex is that the reflex arc contains a minimum of elements - muscle spindles generate impulses that pass into the spinal cord and cause monosynaptic excitation in α-motoneurons of the same muscle. - Tendon, various tonic and rhythmic reflexes
. - In quadrupeds, an extensor impulse
.
Epidemiology
Despite the fact that the true incidence of FMS is unknown, and the number of patients with classic FMS caused by anomalies of the filum terminale, according to some data, does not exceed 0.1% in the pediatric population (Bademci G., 2006), only in the USA during the period From 1993 to 2002, more than 9,000 operations were performed to eliminate spinal cord tethering (Lad SP, 2007).
SFM manifests itself during periods of accelerated child growth
According to most authors, FMS is characterized by a progressive course , while the age at which deterioration of the condition may occur varies from 8 months to 16 years and does not always correspond to periods of accelerated growth. It is noted that at older ages, the manifestation of FMS may be associated with physical activity or injury (Pang D., 1982)
During the first year of life, a child grows by an average of 20 cm due to lengthening of the body
Experimental data suggest that timely removal of fixation can lead to restoration of spinal cord function (Yamada S., 1981; Schneider SJ, 1993). However, it has been shown that sudden excessive stretching of the spinal cord can lead to irreversible structural changes (Kocak A., 1997). In this case, in contrast to gradual long-term stretching (Pfister BJ, 2004), the formation of a persistent neurological deficit is most likely due to rupture of the spinal cord pathways (Yamada S., 2003).
In this regard, concern is raised by the fact that children suffering from FMS often remain under the supervision of orthopedists and urologists for a long time without receiving appropriate neurosurgical treatment. This can lead to the formation of irreversible neurological and orthopedic deficits, as well as persistent pelvic disorders
Spinal cord structure
In the center of the white cord there is a canal surrounded by gray matter. This is the name given to a bundle of nerve cells that looks like a butterfly. This is due to the presence of anterior and posterior horns. They can also be found on the side.
The axons of those neurons that form the roots are close to the latter. They are responsible for transmitting signals from peripheral parts. The anterior horns contain motor neurons, while the middle horns consist of intercalary cells. The function of an intermediary is performed by dendrites.
Sensory and motor roots (bundles of fibers) are combined in the intervertebral foramina. As a result, mixed nerves are formed, which subsequently form three-neuron plexuses (arches). Their length depends on where the organs between which there is a connection are located. The beginning of each of them is at the level of the corresponding vertebra.
In addition to the fibers, the channel contains a special liquid. The liquor is regularly updated. This is what is taken during the puncture. It is necessary to assess the functions of the spinal cord. The results obtained during laboratory testing are used to make a diagnosis.
Pay attention to the external characteristics of the composition. Turbid and heterogeneous synovial fluid indicates the presence of pathological changes
Normally, biological material should be homogeneous and transparent. The general condition of the cerebrospinal fluid is determined using a microscope.
A schematic plan for eliminating physiological and morphological disorders is drawn up, focusing on the indicators identified during the diagnostic examination.
The main elements include white matter. It forms pathways that connect the segments (there are 31 in total).
Each of them belongs to one of four departments, the list includes:
- cervical – provides motor activity of the elbow joints and diaphragm;
- sacral – responsible for erection, defecation and urination;
- thoracic – transmits signals to the organs of the digestive system, muscles of the back and torso, heart, lungs;
- lumbar - its functions include normalization of the ureters, prostate gland, uterus, kidneys, adrenal glands and bladder.
The morphofunctional characteristics of the segments differ. It should be noted that the spinal part of the central nervous system continues with the medulla oblongata.
Clinical diagnosis
Timely detection of FSM at the stage of the disease when the pathological process is still reversible is facilitated by alertness regarding the presence of skin stigmas of dysembryogenesis and musculoskeletal deformities in newborns (Aldana PR, 2009).
Skin manifestations are detected in 40% of children with FSM. These include local hypertrichosis (“faun’s tail”), subcutaneous lipoma, cutaneous angioma, age spots, rudimentary skin growths in the lumbosacral region.
Skin “stigmas” of dysembryogenesis: hypertrichosis, subcutaneous lipoma, cutaneous angioma.
Congenital musculoskeletal deformities in the form of shortening, hypotrophy of the lower extremities, and foot deformities may attract attention Asymmetrical deformations are considered characteristic of FSM - “hollow”, equinovarus foot, “trigger-shaped” toes, etc. Although asymmetry of the lower extremities is often detected with FSM, pronounced hypotrophy of one of the lower extremities (“stork leg”) is rare (Lagae L., 1990 )
“External” manifestations of FSM in children
FSM is manifested by progressive pelvic disorders (incontinence or retention of urine and feces). Parents may experience constant leakage in the child, as well as frequent constipation or episodes of stool.
At an older age, you can find out whether the gait has changed, whether awkwardness has appeared when walking or running, whether the sole of the shoe has changed over time, in which direction it has become thinner (outward or inward) (Elikbaev G.M., 2008). There may be pain when turning the body, flexing or extending. In pre-adolescence, the main clinical manifestations of FMS are weakness in the legs, gait disturbances, urinary incontinence, and newly developed foot deformities (Yamada S., 2004).
Clinical symptoms of FSM
Adolescents with FSM are characterized by progressive scoliotic deformity of the spine, as well as urinary incontinence, mainly in the form of episodes of gelastic incontinence, often interpreted as a manifestation of a urinary tract infection.
During periods of accelerated growth, spinal deformity may be the main complaint (Trivedi J., 2002). In this case, the development of deformation of one of the feet (hollow foot, clubfoot) on the side opposite to the arch of scoliosis, with a high degree of probability, should be interpreted as a manifestation of FSM (Yamada S., 2001).
When assessing neurological examination data, it is noted that FMS most often occurs in the form of asymmetrical, uneven sensory and motor disturbances, accompanied by a decrease in tendon reflexes, as well as pelvic disorders of a mixed type (Yamada S., 2004). However, in some cases, gross trophic disorders may be detected, accompanied by a violation of the integrity of the skin - trophic ulcers (Nikolaev S.N., 1996; Brand N., 1996).
Disorders of the function of the pelvic organs are detected in 40% of patients with FMS, being its only clinical manifestation in 4% of cases (Prityko A.G., 1997; Metcalfe PD, 2006). The majority of patients with FMS show signs of bladder detrusor hypotension due to damage to the parasympathetic micturition center. Depending on the degree of preservation of urethral resistance, pelvic disorders in them occur in the form of constant or periodic urinary incontinence with the accumulation of varying volumes of residual urine, which in some cases requires catheterization of the bladder (Khachatryan V.A., 2009). Most often, such urodynamic disorders are accompanied by chronic constipation and stool loss due to decreased tone of the rectal sphincters (Kayaba H., 2003).
A decrease in the volume of filling of the bladder against the background of an increase in the tone of its muscles is observed less frequently and may be a consequence of damage to the spinal cord above the level of the conus. In this case, there is a disconnection of the structures of the segmental apparatus and supraspinal centers. Hyperexcitability of the detrusor, manifested clinically in the form of imperative, false urges, is caused by irritation of the bladder wall while maintaining a sufficient number of functioning neurons of the parasympathetic sacral center. Often, in children with FSM, against the background of urodynamic disorders signs of chronic urinary tract infection , vesicoureteral reflux and ureterohydronephrosis are revealed (Elikbaev G.M., 2008).
Thus, the clinical diagnosis of FSM is based on the identification of skin stigmas of dysembryogenesis, musculoskeletal deformities, weakness, sensory disturbances in the lower extremities, as well as pelvic disorders.
Instrumental diagnostics
Currently, MRI has almost completely replaced SCT myelography from the diagnosis of FMS, leaving ultrasound as a useful diagnostic method in newborns. Along with this, it is believed that the selection of candidates even for this non-invasive study should be based on a thorough clinical examination.
Previously, it was believed that all children with FSM have spina bifida (Boone D., 1985), but modern studies show that FSM also occurs in the absence of spina bifida (Ackerman LL, 2003). Thus, despite the fact that the occurrence of spina bifida occulta in the population is about 22%, only a small part of them have FSM (Nejat F., 2008).
It is known that during the period of intrauterine development, the growth of the spinal column outstrips the increase in the length of the spinal cord, forming a difference in length and a higher end of the spinal cord. The difference in their length increases most actively from the 12th to the 20th week of gestation, slowing down subsequently (Zalel Y., 2006).
It was previously believed that in newborns the spinal cord ends at the level of the L2-L3 vertebral disc, and then migrates cranially and reaches the level of the L1-L2 vertebrae by two months (Barson AJ, 1970). Subsequent studies showed that in most cases it ends at the level of the L2 vertebrae at birth (Wolf S., 1992; Robbin ML, 1994; Hill CA, 1995).
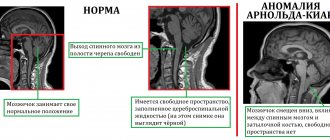
The level of the end of the spinal cord can normally vary from the middle of Th12 to the middle of the L3 vertebra, but in 94-98% of cases it is above the level of the L2-L3 vertebral disc (Reimann AF, 1944). Taking these data into account, the idea was formed that when the conus of the spinal cord is located below the level of the L2 vertebra, we can talk about radiological signs of SFSM. However, a low position of the conus spinal cord can be observed in some healthy newborns (Hughes JA, 2003; Thakur NH, 2011), and FSM can develop even with a normal position of the conus spinal cord (Warder DE, 1993).
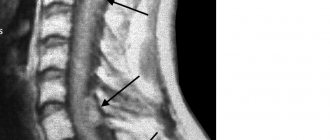
Low location of the conus of the spinal cord in various forms of spinal dysraphism
End thread
Normally, the spinal cord ends in a filum terminale, which is a fibrovascular cord of parallel collagen bundles connected by a transverse network of collagen and elastin fibers and capillaries located between them (Fontes et al 2006). The diameter of the terminal filament normally ranges from 1.1 to 1.2 mm (Yundt KD, 1997). When its thickness is more than 2 mm, it was proposed to consider the diagnosis of FSM justified.
The filum terminale of the spinal cord is normal (left) and pathological (right)
However, thickening of the filum terminale, as it turned out later, is not a prerequisite for the development of FSM (Pinto FC, 2002), and its clinical manifestations can develop with a smaller diameter of the filum terminale (Nkhazar GB, 1995; Selden NR, 2006). Also, fatty infiltration of the filum terminale, which is normally detected in 5.8% of subjects (McLendon RE, 1988), is not a mandatory sign of the presence of FSM.
When fixing the spinal cord, the filum terminale is most often located behind the roots of the cauda equina, and the spinal cord is deviated posteriorly and adjacent to the arachnoid membrane (Fitz CR, 1975).
The tensioned final thread (indicated by the arrow) fits tightly from the inside to the thinned dura mater
As methods of additional differential diagnosis, MRI in the prone position, as well as standing with the torso flexed and extended (functional MRI), was proposed, but so far they have not become widespread (Levy LM, 1988; Zamani AA, 1998; Witkamp T.D., 2001; Niggemann P., 2011; Singh S., 2012).
In the prone position (image on the right), the dorsal deviation of the conus and filum terminale of the spinal cord remains.
The experience of performing DWI tractography in patients with FSM allowed us to assume its role in terms of differential diagnosis of spinal cord structures and scar tissue, and also allowed us to put forward the assumption that, based on the indicators of fractional anisotropy, one can judge the degree of preservation of the spinal cord conductors and evaluate the prognosis of surgical treatment of FSM (Filippi CG, 2010).
DWI tractography of the spinal cord and cauda equina roots (right)
Thus, none of the previously proposed neuroimaging criteria for diagnosing FMS is currently absolute.
Data from electrophysiological examination methods are also not believed to be of decisive importance in the diagnosis of FMS (Yamada S., 2007). In particular, the study of somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs) in children is often uninformative, and the traditionally considered electroneuromyogram (ENMG) patterns, despite the identified clinical picture of SPS, may be within normal limits (Gusev A.R., 1990) . It is reported that registration of SSEPs from the tibial nerves can be used as a screening method of examination, as well as to assess the long-term results of surgical treatment of SSEP in adults (Li V., 1996; Kale SS, 1998).
It should be noted that many authors point to the importance of urodynamic examination data in diagnosing FMS, including in cases of relapse of spinal cord fixation (Meyrat BJ, 2003; Metcalfe PD, 2006; Rendeli C., 2007; Maher CO, 2009).
Anatomical features
A rather thick tourniquet, white in color, located in the spinal canal - this is the human spinal cord. Its diameter is about 1-1.5 cm, and its length almost reaches half a meter (up to 45 cm). This organ weighs about 38 g.
The narrow spinal canal is not only the location of an important organ, but also its protection. The core of the organ consists of a gray substance
It is covered by a white substance, which is also covered with protective and nourishing shells for the core. This is the general plan of the structure of the spinal cord.
Topography
The structure and functions of the spinal cord are quite complex. Neurosurgeon students study it in detail. Experts very carefully consider the development of the spinal cord. Ordinary people are interested in the question of what its topography is and familiarity with the leading role of this organ.
So, it is quite simple to describe the essence and goals that this body serves. The cervical spinal cord at the level of the back of the head in the area of the foramen passes into the cerebellum. The spinal cord ends at the level of the first 2 lumbar vertebrae. The conus spinal cord is located where a pair of vertebrae are located in the lumbar region. Next is the well-known “terminal thread”.
But this fragment is considered atrophied. It is called the “end” region. Nerve endings called “roots” are distributed along the entire circumference of the thread. The filum terminale is equipped with a substance containing a small proportion of nervous system tissue. But the outer part is not even equipped with a similar fabric.
The topography of the organ includes a pair of thickenings where the innervating processes emerge (cervical thickening of the spinal cord and lumbar). The outer and rear surfaces of the bundle are separated by slits called “middle”. The one in front is deeper, the back one is smoothed.
External structure
The general structure of the spinal cord suggests its division into a number of surfaces: posterior, anterior and two lateral. The spinal cord has faint grooves on the lateral surface. They are located longitudinally, and nerves extend from the grooves. They are also called “roots”. In the lumbar area, together with the terminal filament, they form a tail, which is commonly called a horse's tail. The grooves divide half of this cord into the following structures:
- front;
- lateral;
- posterior (cords).
The grooves of the spinal cord extend along the canal. The roots are divided into anterior ones - they are formed by efferent neurons, and posterior ones, created by afferent neurons. Their bodies converge into a knot. The roots unite and form a nerve. So, on all sides of the tourniquet there are over 30 nerve endings, forming exactly the same number of pairs. This is the external structure of the spinal cord.
White matter
All cords are made entirely of white matter of the spinal cord. They consist of longitudinal nerve fibers. These threads converge, forming peculiar conductors. Based on their functional purpose, fibers are divided into 3 types:
- motor;
- associative;
- sensitive.
The first are represented by short bundles and combine all parts into a single system. The second ones are called ascending. They give signals to the centers. Still others are descending. They provide signals from central structures to areas of the horns.
Gray matter
It structurally resembles grouped longitudinal plates consisting of homogeneous neurons. It contains not only neuronal bodies, but also neuropil, glial cells and capillaries. Along the entire spine it forms 2 columnar types, left and right. They are connected by gray adhesions.
The anterior horns contain the largest neurons. They form the motor nuclei of the spinal cord and inhibitory neurons. The structure of the gray matter of the background horns is not the same. It contains a huge number of intercalary type neurons.
The lateral horns of the spinal cord fill the centers of the ANS, the dilation of the pupil, the bases of innervation of the digestive system and other important organs of the human body. In the nucleus of the gray matter of the spinal cord there is a canal that neurosurgeons call “central.” It is filled with liquor. In adults, in some places it is filled with cerebrospinal fluid, and in others it is overgrown.
Shells
Anatomy of the spinal cord describes the membranes of the spinal cord:
- vascular soft;
- hard;
- avascular or arachnoid.
The characteristics of shell 1 are as follows: soft, penetrated by vessels and nerves. It is enveloped by the avascular part. There is some space here called “subarachnoid”. The cerebrospinal fluid generated in one of the systems flows into this niche. The last shell is made up of connective tissue; it is strong and flexible. The membranes of the spinal cord and brain are identical and form a single structure.
From the editor: Causes, diagnosis and treatment of goosebumps
Surgery
The polyetiology of FSM, according to a number of experts, often leads to an unreasonable expansion of indications for its surgical treatment (Kulkarni AV, 2004). In this regard, the indications for surgery were proposed to be considered justified with a combination of characteristic radiological and clinical manifestations of FMS and the progressive course of the disease (Drake JM, 2007; Wykes V., 2012). Along with this, most researchers note the predominantly reversible nature of the changes during early correction of SFM (Yamada S., 2007) and the rare improvement in condition after surgery in patients with long-term symptoms (McLone D., 1997; Iskandar BJ, 1998; Haro H., 2004; Hajnovic L., 2007). It should also be noted that in patients considered “asymptomatic”, signs of the development of FMS may include undiagnosed changes in gait, scoliosis, as well as urodynamic disorders that occur under the guise of a chronic urinary tract infection (Sanchez T., 2014).
Previously considered appropriate expectant management was apparently due to the risk of general anesthesia, as well as the conditions of manipulation on the relatively small and seemingly more vulnerable structures of the spinal cord in children. The development of anesthesia, microsurgical equipment and intraoperative neurophysiological monitoring, of course, made it possible to successfully perform surgical interventions at any age immediately after introscopic verification of pathology, even with an asymptomatic course of the disease (Hoffman HJ, 1985; Tamaki N., 1988; Kanev PN, 1990; Koyanagi I., 1997; Schoenmakers MA, 2003; George TM, 2005; Rinaldi F., 2005). Thus, at present, an idea has formed about the advisability of early preventive surgical treatment of FSM, aimed at preventing possible irreversible damage to the spinal cord. It seems especially relevant to eliminate fixation in adolescents before the onset of a period of accelerated growth.
Surgical treatment of FSM consists of eliminating caudally located fixation factors, in particular: pathologically altered filament terminalis (fatty degeneration, thickening, shortening, decreased elasticity), pathological formations of the spinal canal (lipoma, dermoid, dermal sinus, bone, fibrous, septum), and also cicatricial and arachnoid adhesions (Caldarelli M., 2013):
Transplantation of a pathologically altered filament terminale
Removal of conus lipoma
Dermoid cyst removal
Elimination of diastematomyelia
Preference is given to “organ-saving” techniques - laminotomy, microsurgical techniques. As additional options, a number of authors report the effectiveness of using a CO2 laser and an ultrasound aspirator for tissue dissection, indicating minimal energy dispersion and minor traumatic effects of laser and ultrasound compared to traditional methods (McLone DG, 1986; Browd SR, 2009), as well as the feasibility of intraoperative neurophysiological monitoring (Sala F., 2002).
Operating room view Operating microscope
Intraoperative neurophysiological monitoring
Due to the fact that the incidence of neurological loss during surgical treatment of FSM is on average 10.9% (Choux M., 1994), various methods of intraoperative neurophysiological monitoring aimed at identifying and assessing the function of excitable conductive structures ( roots) of the spinal cord . For this purpose, we combined registration of somatosensory, motor evoked potentials, bulbocavernosus reflex, as well as electromyography (free-run EMG) and stimulation electroneuromyography (DNS) from the muscles of the lower extremities and the external sphincter of the rectum. According to the literature, monitoring of evoked motor potentials in combination with stimulation electroneuromyography demonstrated the greatest information content (Paradiso G., 2006).
Multimodal intraoperative neurophysiological monitoring
Features of neurophysiological monitoring in children with FMS are due to impaired formation and immaturity of the spinal cord, as well as its stretching, deformation and ischemia. Against this background, registration of evoked potentials and the bulbocavity reflex is extremely difficult (Rodi Z., 2001), and the main method of neuromonitoring remains stimulation electroneuromyography, with the goal of electrical stimulation mapping of the spinal cord roots (ESC) (Deletis V., 1992).
Electrical stimulation mapping of the spinal cord roots
Despite the known threshold current values for the anterior and dorsal roots of the spinal cord, their values for functionally significant structures in conditions of spinal cord malformations have not yet been established. Analogies with the results of studies in which, when stimulating the filum terminale, the voltage required for a muscle response exceeded that when stimulating the motor root by 100 times (von Koch CS, 2002), it was not possible to draw, since when the root is involved in a mass of adipose tissue or scar the magnitude of the threshold irritation can increase significantly. This is believed to be due to excitation dissipation or partial loss of conductive properties by the spine.
Interesting Facts
This organ has not yet been fully studied - it still hides many secrets from doctors, and their solution in the future may lead to the cure of currently incurable diseases of the nervous system. Here are some interesting facts about this organ:
- If the spine grows over 20 years, then the spinal cord only grows for 5 years.
- Stress leads to a serious decrease in the number of neurons. If the normal number of neurons is 13-14 million, then as a result of stress their number drops by half - this is especially true for pregnant women.
- In the process of evolution of vertebrate organisms, the spinal cord appeared first, and only then the brain. The first performed all the simplest functions, including reflex ones.
- Some living beings are able to live after losing their brain, remaining only with their spinal cord.
- Damage to a specific area of the organ not only causes loss of sensation below the rupture site, but also the ability to sweat. This forces people with injuries to spend more time in the shadows, as the body has partially lost its thermoregulatory function, which is extremely important for life.
- Scientists have not yet come to a general conclusion, and cannot establish the mechanism of hair loss throughout the body in people with spinal cord injuries.
- If the thoracic organ has been affected, the person may lose the ability to cough.
- A biopsy and analysis of the white matter of an organ can detect hundreds and thousands of human diseases.
- The spinal cord very sensitively senses the rhythm of music, and therefore is automatically able to send signals that will make the body move to the rhythm.
- People with a healthy spine are much more active in their sexual life.
The organ is located in the spinal canal, and its length is no more than 45 cm, which is less than the length of the spine itself. This is due to the fact that the brain grows only until the age of five, and the spine, as a rule, until the end of puberty.
This organ controls absolutely all motor processes in the body, including contraction of the heart muscles, breathing and movement of the limbs.
We study the anatomy of the spinal cord:
Location of the spinal cord and its functions:
Thus, the loss of certain functions, for example, leg movements, makes it possible to determine which part was damaged. Injuries to this organ are among the most serious, and the damage is often irreparable. The main thing is to monitor the health of your spine and not overload it unless absolutely necessary.
Related article: How we are made
Latent tethered spinal cord syndrome
One of the pressing problems of pediatric neurosurgery is the choice of optimal treatment tactics for latent tethered spinal cord syndrome, in which, despite the characteristic clinical picture of FMS, there are no such criteria as low location of the spinal cord conus (below the level of the L2 vertebral body), as well as thickening ( more than 2mm in diameter) and/or shortening of the terminal thread
Patient with hidden SFM, conus spinal cord at the L1 vertebral level
Considering that the observational groups of latent FSM described in the literature are relatively small, to date there has been no consensus regarding the optimal treatment tactics for this pathology. Most experts point to the advisability of excision of the terminal filament and provide data on the high effectiveness of this manipulation, while a number of authors, on the contrary, express doubts about the existence of sufficient indications for surgical intervention. Their skepticism is based, among other things, on the fact that urinary disorders in children, which is considered the most characteristic sign of latent FMS, are associated with stretching of the conus of the spinal cord in no more than 1.5% of cases, and its true causes are other pathologies.
When the torso is flexed, the height of the spinal canal increases by more than 7%. In this case, damping of the longitudinal tension transmitted to the spinal cord is ensured due to the elastic properties of the final thread, caused by a balanced combination of elastin, collagen and reticular fibers. One of the possible reasons for the development of latent FSM is considered to be invasion of adipose tissue, sclerosis and dystrophic changes, as a result of which the elasticity of the terminal filament decreases. With insufficient compensation for stretching, it is transmitted to the caudal parts of the spinal cord, and with a further increase in vertebromedullary disproportion, it spreads to the higher segments of the spinal cord. The formation of a detailed clinical picture of FMS can take a long period of time and be remitting in nature against the background of periods of slowed growth and reflex limitation of motor activity, while some of the formed motor, sensory or orthopedic deficits may be irreversible. It is obvious that early diagnosis and timely correction of latent FSM has a significant impact on the prognosis of the disease. In the case of a combination of pelvic disorders and/or pain syndrome that are resistant to drug therapy, as well as in the presence of characteristic neurological and orthopedic disorders in children with spina bifida occulta, in our opinion, there are indications for excision of the filum terminale, even with a normal location of the caudal parts of the spinal cord.
Electrostimulation identification and intersection of the tense terminal filament with hidden SFSM
Hypoglossal nerve (XII) - Anterior branch
1.
HYPOGLOUS NERVE (XII)
, n. hypoglossus. Twelfth cranial nerve. It leaves the brain through numerous roots between the pyramid and the olive, passes through the canal of the same name and descends between the internal jugular vein and the internal carotid artery. Above the posterior edge, the m.mylohyoideus enters the muscles of the tongue. Rice. B.
2.
Lingual branches
, rami linguales. They arise from the trunk of the hypoglossal nerve and innervate the stylohyoid, hypoglossus and genioglossus muscles, as well as the muscles lying inside the tongue. Rice. B.
3.
Radicular threads
, fila radicularia. They emerge from the spinal cord and join into bundles that form the anterior and posterior roots of the spinal nerve. Rice. A.
5.
Anterior root (motor)
, radix anterior (motoria). Contains axons of neurons in the anterior and lateral columns of the gray matter of the spinal cord. Rice. A.
6.
Spinal (sensitive) node
, ganglion spinale (sensorium). Located in the intervertebral foramen as part of the dorsal root near the junction with the radix anterior. Consists of pseudounipolar neurons. Rice. A.
8.
Spinal nerve trunk
, truncus nervi spinalis. It starts from the junction of the anterior and posterior roots and continues to the level of origin of the first branch. Rice. A, Fig. IN.
9.
Anterior branch
, ramus anterior. The largest branch of the spinal nerve. Connecting with the anterior branches of adjacent spinal nerves it forms plexuses. The anterior branches of the thoracic nerves are called intercostal nerves. Rice. A.
From the editor: Game exercises for the development of memory in primary schoolchildren
10.
, ramus posterior. A smaller branch of the spinal nerve, compared to the anterior one, which innervates the skin and autochthonous muscles of the back. Rice. A.
11.
Meningeal branch
, ramus meningeus. Contains sensory and sympathetic fibers. It passes in front of the spinal nerve, enters the spinal canal through the intervertebral foramen and, together with other meningeal branches, forms a plexus that innervates the membranes of the spinal cord. Rice. A.
13.
Ponytail
, cauda equina. It is formed by the roots of the caudal spinal nerves (starting from the 1st and 2nd lumbar) and the filum terminale. Rice. IN.
14.
Medial branch
, ramus medialis. Each of them begins from the posterior branch of the corresponding spinal nerve. Contains motor and sensory fibers. Rice. A.
17.
Suboccipital nerve
, n. suboccipitalis. Posterior branch of the 1st cervical spinal nerve. Passes between the vertebral artery and the posterior arch of the atlas, innervates the short suboccipital muscles. Rice. G.
19.
Greater occipital nerve
, n. occipitalis major. Posterior branch of the 2nd cervical spinal nerve. First it passes between the axial vertebra and the inferior oblique muscle of the capitis, then pierces the trapezius muscle and goes to the semispinalis capitis muscle and the skin of the back of the head. Rice. G.
20.
Third occipital nerve
, n. occipitalis tertius. Posterior branch of the 3rd cervical spinal nerve. Innervates the skin of the posterior neck near the midline. Rice. G.
21.
CERVICAL PLEXUS
, plexus cervicalis. Formed by the anterior branches of the first four cervical spinal nerves (C 1 - 4). Innervates the skin and muscles of the neck.
23.
Cervical (hyoid) loop
, ansa cervicalis (hypoglossi). It consists of fibers from the hypoglossal nerve and the first three cervical spinal nerves. Innervates the hypoglossal muscles. Rice. B.
24.
Anterior root
, radix anterior. It arises from the hypoglossal nerve and contains efferent fibers of the first to third segments of the spinal cord. Rice. B.
25.
Lesser occipital nerve
, n. occipitalis minor. The most superior cutaneous branch. It rises along the posterior edge of the sternocleidomastoid muscle to the skin of the back of the head, lateral to the n.occipitalis mayor. Rice. G.
28.
Greater auricular nerve
, n. auricularis magnus. It crosses vertically the middle of the sternocleidomastoid muscle and goes to the auricle. Rice. G.
29.
Anterior branch
, ramus anterior. Innervates the skin over the parotid gland between the anterior surface of the auricle and the angle of the lower jaw. Rice. G.
Results and forecast
It is believed that surgical treatment of FSM allows to achieve improvement or stabilization of the condition of 70-80% of patients (Herman JM, 1993; Sarwark JF, 1996; Koyanagi I., 1997; Sharif S., 1997; Lee GY, 2006; Lad SP, 2007; Bowman RM, 2009; Ostling LR, 2012). According to various data, pain syndrome regresses in most cases (Maher CO, 2007; Bowman RM, 2009; Romagna A., 2013), while improvement in motor function is observed in no more than 80% of patients. According to JM Herman et al., within a period of up to 4 years after surgery, the condition of 93% of children operated on for MMC and 100% of children operated on for spinal lipomas improved or stabilized (Herman JM, 1993).
Short-term and long-term results of surgical treatment of children with FMS at the Pediatric Neurosurgery Clinic of the Russian Scientific Research Institute named after. prof. A.L. Polenova
A decrease in spinal deformity as a result of correction of the spinal spinal cord is observed in 20-50% of cases, and its stabilization in 10-20% (Herman JM, 1993; Bowman RM, 2001). At the same time, it has been noted that when the Cobb angle of deformation is more than 40°, eliminating fixation, as a rule, does not lead to improvement (Pierz K., 2000; McGirt MJ, 2009). There are also descriptions of observations in which scoliosis progresses in patients with MMC at the level of the thoracic spine, despite the elimination of fixation (Reigel DH, 1994).
Regarding the function of the pelvic organs, the authors’ data differ significantly. Their improvement is reported (in 30-60% of cases), including after repeated interventions (Herman JM, 1993; Bowman RM, 2001; Metcalfe PD, 2006; Tarcan T., 2006; Abrahamsson, K., 2007; Maher CO, 2009), and about the absence of changes compared to the preoperative level (Fone PD, 1997). Improvement in capacitance characteristics is most expected in patients with urodynamic disorders such as an “overactive” bladder (Khoury AE, 1990; Nogueira M., 2004; Guerra LA, 2006).
The development or increase in neurological deficit after surgery has been established, according to various sources, in 9.5-35% of cases, more often with repeated interventions (Herman JM, 1993; Albright AL, 1999; Wang B., 2002). We are mainly talking about urological complications (2.2%) (Lad SP, 2007). O are less common than other undesirable consequences of surgery (cerebrospinal fluid leak, meningitis, etc.) (Cochrane DD, 1998; Al-Holou WN, 2009; Bowman RM, 2009;) and are most expected during repeated interventions (Maher CO, 2007). The development of postoperative wound complications is most expected in patients operated on for myelomeningocele, which is apparently due to impaired soft tissue trophism and unsatisfactory wound consolidation. The likelihood of meningitis increases significantly with the formation of a cerebrospinal fluid fistula. When detecting liquorrhea in these patients, hydrocephalus or shunt dysfunction should first be excluded (Rostotskaya V.I., 1962; Orlov Yu.A., 1993; Khachatryan V.A., 1995; Hudgins RJ, 2004). Other complications include failure of the sutures and dehiscence of the surgical wound, which occur in 7-10% of cases (Bowman RM, 2009).
Repeated (recurrent, scar) fixation of the spinal cord
Clinical manifestations of FSM, detected after surgery aimed at eliminating spinal cord fixation, are associated with its re-fixation (Inoue HK, 1994; Colak A., 1998; Ohe N., 2000; Blount JP, 2007; Al-Holou WN, 2009; Yong R.L., 2011; Caldarelli M., 2013). According to the literature, the clinical and neuroimaging (MRI) picture of relapse of spinal cord fixation can develop in 5-50% of operated patients, while reoperation may be required in 30% of them (Herman JM, 1993; Filler AG, 1995; Archibeck MJ, 1997 ; Kang JK, 2003; Morimoto K., 2005; Ogiwara H., 2011).
The main reason for the development of re-fixation is considered to be a local violation of the circulation of cerebrospinal fluid (Kang JK, 2003). This is indirectly confirmed by the high frequency of relapse of fixation after repair of spina bifida and lipomyelocele and relatively low for other anomalies (Caldarelli M., 2013). Apparently, the size and shape of the spinal cord (neural placode), the residual volume of tumor (fatty) tissue, as well as the narrowing of the spinal canal in the anteroposterior direction do not always allow restoration of the spinal cord membranes with the formation of a sufficient volume of subarachnoid spaces for adequate circulation of cerebrospinal fluid in the terminal cistern (Hudgins RJ, 2004), as a result of which the spinal cord fuses with the membranes, and sometimes with scarred soft tissues, spreading beyond the dural sac and even the spinal canal (Caldarelli M., 1995).
Relapse of spinal cord tethering is the second most common cause of deterioration in the condition of patients with myelomeningocele , with a progressive deterioration of 60% of them observed during a follow-up period of up to 5 years (Phuong LK, 2002; Talamonti G., 2007). It is noted that with incomplete elimination of fixation, the relapse rate can reach 80% (Huttmann S., 2001), while in 10% of cases three or more operations may be required to stabilize the condition of patients (Al-Holou WN, 2009; Maher CO, 2007, 2009). According to research results, an increase in the detection of relapse of FSM occurs at ages from 2 to 4 and from 8 to 11 years, regardless of the primary pathology (Herman JM, 1993; Caldarelli M., 1995).
Considering that surgical intervention at the initial stages of the pathological process, according to various authors, leads to improved treatment results, it was proposed to consider a progressive deterioration in the child’s condition or the appearance of new symptoms as indications for surgery . (Bowman RM, 2001; George TM, 2005). Along with this, making a decision about surgical treatment is extremely difficult in chronically “symptomatic” patients with persistent neurological deficits. At the same time, emphasis is placed on the need to exclude other causes of deterioration of the patient’s condition, such as shunt dysfunction, Chiari malformation, syringomyelia, etc. (Bowman RM, 2001; Talamonti G., 2007). It is emphasized that relapse of FMS can occur with erased, slowly progressing symptoms, which also complicates the recognition of the pathological process and is the reason for its late diagnosis . It is reported that the most common clinical manifestation in older age is progressive spinal deformity (Carstens C., 1996). However, other signs are fair to consider contractures of the lower extremities, deterioration of gait, and back pain (Herman JM, 1993; Bowman RM, 2009). Thus, the clinical picture of recurrent FSM is in many ways similar to that of primary FSM. At the same time, the onset of the disease is often associated with pain, and the deterioration of the condition with progressive spinal deformation (Caldarelli M., 1995).
Surgical treatment for re-fixation of the spinal cord is aimed at eliminating tension and deformation of the spinal cord and roots, as well as restoring cerebrospinal fluid circulation in the fixation area.
Elimination of scar fixation of the spinal cord
The area of scar adhesions during re-fixation may depend on the method of wound closure during primary intervention (Caldarelli M., 2013). In cases where neural tube reconstruction was performed during the primary operation, the scar is usually located in the midplane over a limited area (Caldarelli M., 2013).
Thus, the main measure to prevent re-fixation of the spinal cord during surgical correction of MMC and spinal lipomas is considered to be reconstruction of the neural placode (Pang D., 2010; Talamonti G., 2007). Expanding plastic surgery of the dura mater with various artificial substitutes is also used (Inoue HK, 1994; Ohe N., 2000). In addition, after surgery, bed rest in a prone position is recommended in order to avoid gravitational contact of the spinal cord with the dura mater.
Spinal cord puncture
A spinal puncture involves inserting a special needle into the subarachnoid space. A puncture of the spinal cord is performed in special laboratories, where the patency of this organ is determined and the pressure of the cerebrospinal fluid is measured. The puncture is performed for both therapeutic and diagnostic purposes. It allows you to timely diagnose the presence of hemorrhage and its intensity, find inflammatory processes in the meninges, determine the nature of the stroke, and determine changes in the nature of the cerebrospinal fluid, signaling diseases of the central nervous system.
From the editor: Causes and prevention of stroke
Often a puncture is performed to administer radiopaque and medicinal fluids.
Indications for spinal cord puncture:
- Meningoencephalitis;
- Unexpected hemorrhages in the subarachnoid space due to rupture of an aneurysm;
- Cysticercosis;
- Myelitis;
- Meningitis;
- Neurosyphilis;
- Traumatic brain injury;
- Liquororrhea;
- Echinococcosis.
Sometimes, during brain surgery, spinal cord puncture is used to reduce intracranial pressure parameters, as well as to facilitate access to malignant neoplasms.
Consultation with a neurosurgeon
neurosurgeon, leading researcher at the Department of Pediatric Neurosurgery, Ph.D. Sysoev Kirill Vladimirovich
author of FEDERAL CLINICAL GUIDELINES FOR THE TREATMENT OF SPINAL BIFIDAS AND FSM IN CHILDREN, approved by the decision of the XXXX Plenum of the Board of the Association of Neurosurgeons of Russia, St. Petersburg, 04/16/2015.
CONTACTS: e-mail
REVIEWS ON SITES: https://prodoctorov.ru/spb/vrach/144441-sysoev/ https://spb.napopravku.ru/doctor-profile/sysoev-kirill-vladimirovich/
The site is for informational purposes only, is not the site of a medical organization, and is not a public offer as defined by the provisions of Article 437 (clause 2) of the Civil Code of the Russian Federation. Interaction with a medical specialist is regulated by the rules of the clinic in which he works.