Diabetes mellitus (DM) is currently a global psychological, social and economic problem. According to IDF (2012), 371 million people worldwide suffer from diabetes. According to WHO forecasts, for the period 2005-2030. The number of deaths from diabetes will double.
An analysis of federal register data showed that the prevalence of type 1 diabetes (T1DM) over 10 years in children increased by 35.7% (from 59.4 to 80.6% per 100 thousand children); in adolescents - by 68.9% (from 108.5 to 183.5% per 100 thousand), in adults - by 2.36% (from 224.5 to 229.8% per 100 thousand) [1] . The main trends in the dynamics of epidemiological indicators of T1DM in children in the Russian Federation are comparable to the average global annual increase in incidence (2.8%) [2].
Although T1DM accounts for only 10% of all patients with diabetes, it is particularly severe and is accompanied by the development of vascular complications. T1DM in childhood and adolescence, already in the early stages, is a risk factor for the development of chronic cerebrovascular pathology and is manifested by both clinical neurological syndromes and subclinical disorders of the central nervous system, detected in neuropsychological, neurophysiological and biochemical studies [3]. The medical and socio-economic significance of this problem can hardly be overestimated, given the prevalence of diabetes and the frequency of damage to the nervous system, which leads to enormous financial costs for the treatment and social security of such patients. The frequency of damage to the nervous system in diabetes correlates with the duration of the disease, its severity and the age of the patients [4]. A significant role is played by early identification of encephalopathy (EP), taking into account the progressive nature of damage to brain structures. Even mild or moderate disorders of the motor, emotional-volitional and cognitive spheres lead to social and everyday maladjustment, which reduces patients’ compliance with medical recommendations. The quality of life (QoL) of patients with diabetes and their families is lower than in the general population [5–7]. Quality of life parameters can become determining factors in an individual’s ability to manage their disease and provide self-control.
The greatest influence on QOL in T1DM in childhood and adolescence is exerted by the psychoemotional characteristics of patients, insulin therapy regimens, and types of attitude towards the disease [8]. The negative impact of T1DM was noted in relation to such aspects of quality of life as freedom of nutrition, work activity, physical capabilities and anxiety about the future. Among the factors mediating the impact of diabetes are compensation of carbohydrate metabolism, complications, patient behavior associated with the disease, frequency of self-monitoring of glycemia, and gender [9].
EP “in its pure form” occurs only in patients with T1DM (in 80.7% of cases), since its development is mainly due to ineffective metabolic control [10, 11]. The term “diabetic encephalopathy” (DE) was proposed by R. DeJong in 1950. DE is understood as persistent cerebral pathology that occurs under the influence of metabolic and vascular disorders, which is clinically manifested by neurosis-like and psycho-like defects, organic neurological and autonomic symptoms. DE includes characteristic biochemical, electrophysiological and morphological changes that can lead to cognitive deficits and significantly reduce the quality of life of both the patient and his loved ones [12]. According to the classification of E.V. Schmidt (1985), EP is a progressive diffuse small-focal brain lesion, manifested by a combination of symptoms of focal lesions and asthenic symptoms. The clinical polymorphism of DE gives reason to assume the existence of at least several pathophysiological mechanisms of its formation. The pathogenesis of DE is associated with two main components - metabolic and vascular. The development of microangiopathy is mediated by the accumulation of LDL in the vascular wall, activation of lipid peroxidation processes, increased formation of free radicals, and suppression of the synthesis of prostacyclin, which has an antiplatelet and vasodilator effect [12, 13]. The progression of microangiopathy leads to a decrease in endoneurial blood flow with the development of hypoxia, which promotes the switching of the energy metabolism of nervous tissue to ineffective anaerobic glycolysis, during which only two ATP molecules are formed from one glucose molecule, while in the reaction of aerobic glycolysis - 38 molecules. As a result, the concentration of phosphocreatinine in neurons decreases and the lactate content increases, which leads to the development of oxygen and energy starvation of the nervous tissue. A decrease in endoneurial microcirculation and worsening dysfunction of nerve fibers contributes to a decrease in the synthesis and increase in the destruction of nitric oxide (NO), which has a vasodilating effect, which can become one of the reasons for the development of arterial spasm, which is an important pathogenetic mechanism for the development of arterial hypertension in diabetes. In addition to the pathogenetic significance of endoneural blood flow disorders, metabolic disorders also play an important role. It has been established that a decrease in the speed of impulse conduction along myelin fibers is due to a pathologically high intra-axonal concentration of Na+ ions, in the development of which the main role belongs to a decrease in the activity of tissue Na+/K+-ATPase, which causes secondary vascular disorders, neurotrophic disorders, neurotoxicosis and, as a consequence, structural changes in neurons, as well as a violation of the speed of excitation along the nerve [12]. DM is a major contributor to the loss of white matter in the brain [13, 14].
There is no doubt about the relationship between acutely or chronically developing changes in the central nervous system with hyper- or hypoglycemia. M.R. Chuiko et al. [16] showed that the clinical manifestations of EP are especially characteristic of patients with frequent hypoglycemic conditions, debuting at the age of 26-35 years. I. Brands [10] in his book “Diabetes and the brain” described one of the pathogenetic causes of cognitive impairment in diabetes, which is hyperglycemia.
Animal models with induced T1DM have shown that insulin deficiency plays an important role in neuronal apoptosis, as well as in the development of degenerative phenomena in the white matter of the brain [17]. The basic mechanisms underlying the complications of diabetes are the activation of the polyol and hexosamine pathways of glucose metabolism, the formation of end products of excess protein glycation, and an increase in the content of various isoforms of protein kinase C. Most of the known metabolic and vascular mechanisms for the development of extra- and intracellular pathology in late complications of diabetes provide overproduction superoxide in mitochondria. Thus, the main reason for the formation of late complications, including EP, is oxidative stress - an imbalance between the production of free radicals and the activity of antioxidant enzymes, which is reduced in diabetes.
To diagnose ES, psychological questionnaires are more often used, which do not exclude subjectivity in assessing the results. Therefore, a search is underway for diagnostic biomarkers of brain damage [10, 17, 31, 32]. Currently, neurospecific proteins (NSPs), which change quantitatively in various diseases, are considered such biomarkers [18]. Among them, the most studied are: neurospecific enolase (NSE) - a marker of neurons, glial fibrillary acidic protein (GFAP), protein S100 (isoforms - S100A1 and S100B) - markers of astrocyte death and myelin basic protein (MBP) - a marker of oligodendrocyte damage. An increase in NBP in the blood indicates damage to the nervous tissue and makes it possible to give an intravital characterization of the state of the central nervous system and assess the dynamics of the neurodegenerative process [19].
In the works of B. Danna et al. [20] there is information about the role of proteins S100A1 and S100B in the development of EC in diabetes. In recent years, the determination of these proteins has been increasingly used to diagnose damage to brain tissue in cerebrovascular accidents. Increased concentrations of S100A1 and S100B correlate with the volume of brain damage. The S100B protein is produced predominantly by brain astrocytes and is a marker of astroglial activation, mediating its effects through interaction with receptors for advanced glycation end products. S100B has been shown to exhibit neurotrophic activity at physiological concentrations and neurotoxic activity at high concentrations. The S100B protein is considered as one of the key molecular components of complex intracellular systems that ensure the functional homeostasis of brain cells by coupling and integrating diverse metabolic processes [21].
Thus, the S100 protein isoforms are the most universal of the known macromolecules involved in the regulation of almost all major membrane, cytoplasmic and nuclear metabolic processes associated with providing mechanisms for the perception and integration of information entering the nervous system [21, 22], in the response of early response, in the implementation of genetic programs of apoptosis and anti-apoptotic protection [23]. Transgenic mice with overproduction of S100B showed defects in hippocampal function and impaired short-term memory, and a partial decrease in the ability to solve spatial problems [24–27]; in addition, their adaptation to a new environment is impaired, but at the same time the reduction of anxiety is enhanced (according to a number of behavioral tests) [27-29, 31]. M. Rothermundt et al. [29] showed that the level of S100B is increased in patients with mild/moderate depression; its serum level also increased in patients with the melancholic subtype of depression, as opposed to the non-melancholic subtype [31]. An increase in the level of S100B in the blood of patients with diabetes can be considered as a result of the development of reactive gliosis and increased permeability of the blood-brain barrier caused by hyperglycemia and cerebral hypoxia. Thus, an increase in the level of S100B is associated primarily with dyscirculatory disorders, which with a high degree of probability confirms the predominant activation of reactive gliosis in diabetes. Consequently, the severity of damage to the central nervous system in this disease is to a certain extent determined by the peculiarities of the reaction of brain structures to hypoxia and metabolic stress, making certain areas of the central nervous system (primarily mesencephalic) the most vulnerable, all other things being equal, especially with ketoacidosis, which is confirmed by E. McIntyre et al. [30], who recommend the use of S100 as a biomarker of brain damage.
O.Z. Puzikova et al. [32] found that the high content of S100B protein in the serum of children and adolescents with diabetes is directly dependent on the severity of cerebral disorders and the level of desaturation during night sleep, as well as on the time of conduction of the auditory impulse in the midline structures of the brain. An inverse relationship was found between the level of this protein and blood flow velocity in the anterior and posterior cerebral arteries, which indicates a connection between enhanced reactive astrocytosis in diabetes with hypoxic, discirculatory processes and deterioration of neurophysiological parameters in the central nervous system. M. Strachan et al. [33] showed that NSE and S100 concentrations may play a prognostic role in the development of neurological deficits. Thus, in 2 out of 3 patients who died due to episodes of hypoglycemia, the concentrations of these markers were significantly increased. M. Hovsepyan et al. [34] found that serum levels of S100B and NSE in T1DM were not significantly different, but there was a significant increase in antibodies to NSE.
Some authors consider the development of dysregulation of the serotonergic, noradrenergic systems, as well as the system of endogenous opioid peptides of the brain, to be the key pathogenetic mechanism of EP. Tomsk scientists [35] obtained results indicating an increase in the level of NSE by 1.64 times and a decrease in serotonin by 1.66 times in the blood of patients with type 1 diabetes compared to controls. The severity of the disorders depended on the degree of compensation of carbohydrate metabolism. The authors [35] suggested that chronic hyperglycemia in T1DM contributes to the formation of conditions for damage not only to vascular endothelial cells, but also to the cytomembranes of brain cells, which is accompanied by the appearance in the blood of some brain-specific peptides, in particular NSE. An increased level of NSE in the blood was evidence of damage to neuronal membranes, indicating dysfunction of brain structures, which worsens with increasing duration of T1DM.
K.A. Pavlov [36] published information on astrocyte intermediate filament protein (GFAP), which is highly specific for the brain and is released only in the event of necrotic cell death and cytolysis. Almost any pathological process in the central nervous system leads to pronounced activation of the astroglial component of the nervous tissue. The most striking manifestation of reactive astrogliosis at the molecular biological level is a sharp increase in GFAP expression in activated astrocytes. Further development of the pathological process leads to the death of reactive astrocytes, as a result of which the resistance of the cell membrane is disrupted, and GFAP ends up in the intercellular fluid, from where it is eliminated into the patient’s bloodstream and cerebrospinal fluid. The appearance of GFAP in biological fluids of the body is possible only when the resistance of the blood-brain barrier is impaired and the vascular endothelium fails [37, 38]. The level of GFAP in biological fluids directly depends on the number of dead or damaged astrocytes, which, in turn, reflects the severity of the neurodegenerative process [39]. E. Coleman et al. [40] showed that streptozocin-induced diabetes in rats increases the level of GFAP in the hippocampus, cerebellum, and white matter. On the other hand, DM-induced in vivo inhibited astrocyte activity and thereby decreased GFAP levels [41]. Fluctuations in the level of this protein depend on the duration of the pathological process [42]. Experimental data on the content of GFAP in various areas of the brain are ambiguous [43, 44]. Thus, the role of GFAP as a marker remains poorly understood.
A marker of brain damage, MBP, is currently being actively studied.
V.P. Chekhonin et al. [45] found high levels of MVR in children with EP. A study by Italian scientists reported for the first time that the content of two MBP isoforms (18.5 and 21.5 kDa) decreases in the spinal cord of rats with streptozocine diabetes [46]. In T1DM, an increase in the level of autoantibodies to MBP of the IgG and IgM classes was observed, which depended on the duration of the disease [47].
Thus, despite the variety of markers of brain damage, their role in the development and progression of EP in diabetes has not been established. Further research is needed to create an algorithm for diagnosing this condition [48–50].
Diabetic encephalopathy
Damage to the nervous system is one of the earliest and most common complications of diabetes. Diabetic encephalopathy refers to the central form of neuropathy in diabetes mellitus.
According to various authors, this pathology occurs in 90-100% of patients with diabetes mellitus (DM). Moreover, in some cases, symptoms from the nervous system precede the appearance of clinical signs of diabetes.
After the discovery of diabetes mellitus as an independent disease in the 18th century and the appearance of the first works on neurodiabetology, interest in the study of the pathology of the nervous system has been steadily growing.
The medical and socio-economic significance of this problem is difficult to overestimate, given the prevalence of diabetes mellitus, the frequency of the development of disabling lesions of the nervous system caused by it with a decrease in the quality of life and social activity of patients, as well as the enormous material costs of treatment and social security for patients. The frequency of damage to the nervous system in diabetes mellitus correlates with the duration of the disease, severity and age of the patients.
In diabetes mellitus, the central nervous system (encephalopathy, myelopathy), the peripheral nervous system (poly- and mononeuropathies), and the peripheral autonomic nervous system (autonomic neuropathy) are affected.
The term “ diabetic encephalopathy ” (DE) was proposed by R. DeJong in 1950. Diabetic encephalopathy was understood as a persistent cerebral pathology that occurs under the influence of acute, subacute and chronic diabetic metabolic and vascular disorders, clinically manifested by neurosis-like and psycho-like defects, organic neurological and autonomic cerebral symptoms.
According to the classification of E.V. Schmidt (1985), encephalopathy is a progressive diffuse small-focal brain lesion, clinically expressed by a combination of symptoms of focal brain damage and asthenic manifestations.
Etiology and pathogenesis of diabetic encephalopathy
Diabetic encephalopathy, like other types of diabetic neuropathy, develops due to widespread damage to neurons and their processes. The extreme clinical polymorphism of diabetic encephalopathy suggests the existence of at least several pathobiochemical mechanisms involved in its formation, between which there are obviously relationships. Research in recent years confirms these assumptions, but detailed study continues.
The pathogenesis of diabetic encephalopathy is traditionally associated with two main directions - metabolic and vascular. At the same time, the undoubted priority is recognized for microcirculation disorders - diabetic microangiopathy.
The development of microangiopathy is associated with the accumulation of low-density lipoproteins (LDL) in the vascular wall, activation of lipid peroxidation (LPO), increased formation of free radicals, and suppression of the synthesis of prostacyclin, which has an antiplatelet and vasodilator effect. The progression of microangiopathy leads to a decrease in endoneurial blood flow.
Developing hypoxia switches the energy metabolism of nervous tissue to ineffective anaerobic glycolysis, during which only two ATP molecules are formed from one glucose molecule, while in the reaction of aerobic glycolysis - 38 molecules. As a result, the concentration of phosphocreatine in neurons decreases, the lactate content increases, and oxygen and energy starvation of the nervous tissue develops.
A decrease in endoneurial microcirculation and aggravation of nerve dysfunction is facilitated by a decrease in synthesis and an increase in the destruction of NO, which has a vasodilating effect. This may be one of the reasons for the development of arterial spasm, which is an important pathogenetic mechanism for the development of arterial hypertension in patients with diabetes mellitus.
Recognizing the pathogenetic significance of endoneural blood flow disorders in the formation of diabetic encephalopathy, the importance of metabolic disorders cannot be underestimated. It has been established that the decrease in conduction velocity along myelin fibers is due to a pathologically high intra-axonal concentration of Na+ ions, in the development of which the main role belongs to a decrease in the activity of tissue Na+/K+-ATPase.
Predetermining factors for the development of diabetic encephalopathy are:
- pathological biochemical processes triggered under conditions of absolute or relative insulin deficiency: activation of the polyol pathway of glucose oxidation, depletion of myoinositol reserves (precursor of phosphoinositol, the main regulator of tissue Na+-K+-ATPase activity);
- an increase in the concentration of free radicals in neurons, limiting lipid peroxidation processes and oxidative stress - the most important factor in damage to nerve cells; irreversible glycosylation of proteins - the inclusion of carbohydrates in the structure of serum proteins, cell membranes, lipoproteins, collagen, neurons, leading to disruption of the functional activity of cells, the formation of autoantibodies to proteins of the vascular walls (an essential factor in the pathogenesis of microangiopathy);
- the formation of a stable HbA1c complex with a low affinity for oxygen, resulting in tissue hypoxia.
All this causes secondary vascular disorders, neurotrophic disorders, neurotoxicosis and, as a consequence, structural changes in neurons and a violation of the speed of excitation along the nerve.
Hyperglycemia is undoubtedly an important factor contributing to the development of diabetic encephalopathy through the various metabolic disorders that it induces. However, convincing evidence of a direct connection between hyperglycemia and diabetic encephalopathy has not yet been obtained. At the present stage of development of neurodiabetology, there is practically no doubt that achieving stable normoglycemia does not allow stopping the progression of pathology of the nervous system. It is assumed that for the development of diabetic encephalopathy, metabolic disorders are a necessary condition, and the basis for their manifestation is a genetic predisposition.
Also in the pathogenesis of damage to the central nervous system in diabetes mellitus and the development of diabetic encephalopathy, dyslipidemia and atherosclerosis, macroangiopathy and arterial hypertension, the mechanisms of pathogenesis of which are closely interrelated, are of great importance.
Clinical picture and diagnosis of diabetic encephalopathy
Diabetic encephalopathy usually develops gradually, often runs subclinically, its manifestations are masked: in young people - by the consequences of acute ketoacidotic episodes, in older people - by cerebrovascular accidents.
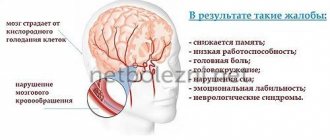
In the clinical picture, first of all, asthenic syndrome is observed (general weakness, increased fatigue, decreased performance, emotional lability, anxiety, impaired concentration, insomnia).
A cephalgic syndrome, manifested by headache, is often detected. At the same time, patients most often describe it as squeezing, squeezing, like a “tight headdress (tension headache), or as a feeling of a heavy head and inability to concentrate (ischemic-hypoxic headache).
Patients almost always experience vegetative dystonia syndrome with the development of vegetative paroxysms, presyncope and fainting. In addition to asthenic and vegetative-dystonic manifestations, focal disorders are found: upper brainstem (anisocoria, convergence disorder, signs of pyramidal insufficiency), vestibular-ataxic syndrome (dizziness, unsteadiness of gait, deviations when performing tests for coordination of movements).
Quite characteristic in the clinical picture of diabetic encephalopathy is the presence of disorders of cognitive functions: impaired memory, attention, slow thinking, apathy, depression, indicating a predominant dysfunction of nonspecific midline structures of the brain. Depression is common in diabetes mellitus: observations show that more than 32% of patients with diabetes mellitus are susceptible to depression. In addition to the impact on general well-being, depression is dangerous due to the loss of control over the course of the disease itself, proper nutrition and the use of insulin. According to experts, one of the reasons for the tendency of diabetic patients to depression is some biochemical changes in the body. Another reason is the constant dependence of people due to illness.
In some patients with diabetes mellitus, hypoglycemic encephalopathy develops as a result of hypoglycemic conditions. Clinically, it is manifested by an increase in lethargy, apathy, adynamia after physical work and on an empty stomach, a disorder of consciousness, most often of the delirium type. The presence of convulsive syndrome is characteristic, pyramidal hemiparesis is possible.
To make a diagnosis of encephalopathy, in addition to asthenic and vegetative-dystonic complaints, it is necessary to establish focal neurological symptoms in the patient. Changes in the electroencephalogram (EEG) in patients with diabetic encephalopathy are characterized as dysregulatory and diffuse in nature (in the form of various “flattening” of the EEG, hypersynchronization rhythms, reductions of the alpha rhythm local and general, inclusions of pathological waves of theta and delta types, changes in EEG reactivity curves, etc.).
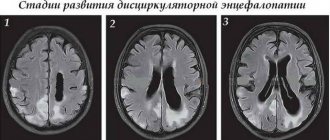
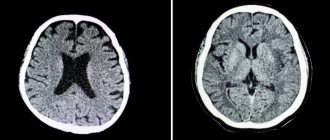
In elderly patients with diabetes mellitus, diabetic encephalopathy is often accompanied by focal neurological deficits, macrostructural changes in the brain (atrophy, post-stroke changes), objectified by computed tomography (CT) and magnetic resonance imaging (MRI), which are a manifestation characteristic of diabetes mellitus and pathogenetically associated macroangiopathies, atherosclerosis and hypertension.
Stroke and transient ischemic attacks from these positions can be considered as manifestations of central neuropathy.
Treatment of diabetic encephalopathy
Treatment of diabetic encephalopathy includes adequate glycemic control, vasoactive and metabolic therapy.
Achieving stable compensation for diabetes is a necessary but not sufficient condition for the prevention and treatment of diabetic encephalopathy. For patients with type 2 diabetes mellitus (DM2), this is especially significant, since in this case tissue metabolic disorders are determined genetically and persist constantly, even under conditions of controlled normoglycemia, and therefore, permanent preventive therapy aimed at improving the metabolism of nervous tissue is pathogenetically justified.
Metabolic therapy. In order to normalize neurometabolic processes in diabetes mellitus, antioxidants have been most widely used in our country and abroad in recent years. Priority in this group of drugs is given to drugs α-lipoic (thioctic) acid (thioctacid, thiogamma, dialipon, espalipon, berlition, thioctodar), which have a powerful antioxidant effect and occupy one of the central places in metabolic therapy. Treatment with thioctic acid, as a result of its positive effect on the main links of pathogenesis, leads to an improvement in the energy metabolism of nervous tissue, an increase in ATP production and transmembrane ion transport due to the activation of mitochondrial oxidative processes, which determines the prospects for its use.
In the treatment of diabetic encephalopathy today, the modeling of new combination drugs, including cerebroprotective effects, in particular piracetam with thiotriazoline, is actively used. The pharmacological effect of thiocetam is determined by the mutually potentiating effect of thiotriazoline and piracetam.
Thiotriazolin has appropriate pharmacological (high antioxidant, anti-ischemic activity), pharmacological (compatibility with other drugs) and pharmacoeconomic characteristics.
We observed 50 patients (36 women and 14 men) aged from 34 to 65 years with both DM 1 and DM 2. Of these, 21 people were with DM 1 and 29 were with DM 2 with concomitant encephalopathy as mixed, and metabolic genesis. Duration of diabetes mellitus from 1 year to 20 years: 10 people had diabetes mellitus - 1-2 years, 12 people - from 3 to 5 years, and 28 people - from 10 to 20 years. All patients received standard complex therapy, which also included thiocetam, which was administered 5.0 ml intravenously for 10 days, then for a month the patients took thiocetam 1 tablet. three times a day for 30 minutes. before meals.
During the observation process, the somatic and neurological status of each patient was assessed, laboratory parameters were monitored in order to assess the overall effect of the drug on the patient’s body (at the beginning and at the end of the study). The clinical assessment of thiocetam was assessed by performing a rheoencephalogram (REG) twice (before the start of treatment and on the 38th day) using the Reocomp computer diagnostic complex; cognitive and mnestic functions were also studied.
Data from a dynamic study of cognitive function indicators revealed the following features. Thus, a statistically significant positive effect of thiocetam on all studied functions was noted: an increase in the “number of lines” indicator by 27% (P < 0.05), a decrease in the proportion of errors by 32% (P < 0.05), an acceleration of the Schulte test by 32 % (P < 0.01).
Analysis of the indicators indicates a positive effect of the drug thiocetam on the main types of memory, and the effect on motor and operational memory is statistically significant, while changes in visual and verbal memory, being positive in quantitative terms, were not statistically significant.
The quantitative characteristics of the positive statistically significant effects of thiocetam are as follows: improvement in motor memory indicators by 43% (P < 0.01), operational, emotional, indirect - by 41% (P < 0.05), for group 2 - 16 and 18%, respectively. (P < 0.05).
In complex therapy of diabetic encephalopathy, vitamins A, C, E, which have an antihypoxic effect, are widely used. Vitamins B1, B6, B12 have neurotropic activity, and it is advisable to carry out repeated courses of treatment at least twice a year. In recent years, new, highly effective forms of these drugs have appeared, in particular, the biologically active fat-soluble form of thiamine (vitamin B1) - benfotiamine, the activity of which is 8-10 times higher compared to water-soluble forms of thiamine, and the ability to penetrate into the nerve cell and convert into the active metabolite of thiamine is even higher.
A combination drug that includes all B vitamins in the required therapeutic dosage – neuromultivit – has become widespread in neurodiabetology. One of the modern highly active drugs is Milgamma 100 (100 mg benfotiamine + 100 mg pyridoxine hydrochloride) for oral administration.
Vasoactive therapy. One of the main places in the treatment of micro- and macroangiopathies is occupied by pentoxifylline (trental), the effect of which is to normalize blood flow at the capillary level by reducing the aggregation of blood cells, reducing its viscosity and increasing the ability of red blood cells to deform. It improves microcirculation, promotes the removal of metabolic products and toxins, increases the volume of circulating fluid and normalizes diuresis. The prolonged form of pentoxifylline allows you to create a longer and more uniform saturation with the drug.
For the treatment and prevention of cerebral circulatory disorders, vasoactive drugs of different groups with a predominantly central effect are used: Cavinton, Stugeron (cinnarizine), Sermion (Nicergoline), Nimodipine (Nimotop), as well as Instenon, a drug with a combined nootropic and cerebral vasodilating effect, and others. Significant importance in preventing the progression of DE is given to the treatment of hypertension and atherosclerosis.
From the monograph “Diabetes mellitus: from child to adult”
Senatorova A.S., Karachentsev Yu.I., Kravchun N.A., Kazakov A.V., Riga E.A., Makeeva N.I., Chaichenko T.V. State Institution “Institute of Endocrine Pathology Problems named after. V.Ya. Danilevsky AMS of Ukraine" Kharkov National Medical University Kharkov Medical Academy of Postgraduate Education of the Ministry of Health of Ukraine