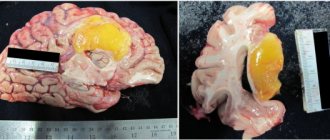
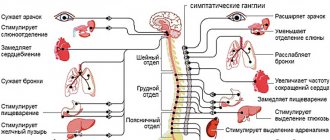
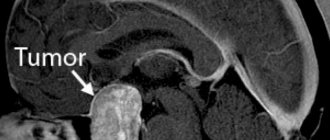
Radiation necrosis , which is a focal structural lesion at the site of the tumor, is a long-term complication of the central nervous system (CNS) after radiotherapy or radiosurgery.
Tissue swelling and the presence of a neoplasm cause changes in the parenchyma of the central nervous system organs located in the tumor bed, which increase the likelihood of developing radiation necrosis. Radiation necrosis can occur when radiotherapy is prescribed for primary central nervous system neoplasms, metastatic brain lesions, or head and neck tumors. Radiation necrosis can occur following any use of ionizing radiation to treat tumors or any protocol for such treatment.
Diagnosis of radiation necrosis and its treatment pose significant difficulties, since the manifestations of this complication of radiotherapy are often overlapped by symptoms of tumor recurrence. An erroneous diagnosis (recurrence of a tumor instead of radiation necrosis) can lead to the prescription of incorrect treatment, which can pose a danger to the patient’s life.
Symptoms of radiation necrosis
It is necessary to understand that radiation necrosis is a long-term complication of radiotherapy, the development of which often occurs months, or even years, after completion of treatment with ionizing radiation. It most often appears between 6 months and 2 years after completion of the last course of radiotherapy.
In modern medical literature, there are data on the incidence of radiation necrosis, which are characterized by significant variability - from 5% to 37% of all patients receiving radiotherapy for intracranial tumors.
However, some authors report that up to 10% of all cases of this complication of radiotherapy were asymptomatic and the detection of radiation necrosis in such cases is entirely due to progress in neuroimaging methods. Attending physicians need to take into account the possible absence of symptoms of radiation necrosis in patients, despite the development of the pathological process.
In cases where the development of radiation necrosis is nevertheless accompanied by symptoms, patients most often experience the following symptoms:
- headache;
- nausea;
- cognitive impairment;
- seizures;
- focal neurological symptoms associated with the localization of the necrosis focus;
- personality change;
- apathy;
- hemiparesis.
Cerebral hemorrhages during radiation necrosis are a rare phenomenon, but, nevertheless, encountered in the practice of neuro-oncologists.
Radiation damage to the skin
Radiation damage to the skin, often called a radiation burn, can have a variety of clinical manifestations.
Radiation damage to the skin (development of radiation burns). Rice. 5. Erythema. Rice. 6 - 8. Development of bubbles. Wet radioepidermitis. Rice. 9. Erosion. Rice. 10. Tripe; dyschromia, telangiectasia and a border of hyperpigmentation are visible.
Erythema - temporary redness of the skin at the site of irradiation; develops on the 13-14th day after single and 2-6 weeks after fractional irradiation. Persistent hair removal develops with single or fractional irradiation of the scalp. Dry epidermatitis develops 7-10 days after a single dose or 2-3 weeks after fractional irradiation. Clinically manifested by erythema, swelling of the skin followed by lamellar peeling. Recovery of irradiated skin is incomplete. The skin remains atrophied, dry, epilated. Later, telangiectasia and uneven pigmentation appear. Wet radioepidermitis is accompanied by sharp redness and swelling of the skin, the appearance of blisters filled with a clear yellowish liquid, which quickly open, exposing the basal layer of the epidermis. After 1-2 days, epithelization begins. Wet epidermitis ends with persistent atrophy of hair follicles, sebaceous and sweat glands, significant thinning of the skin, loss of elasticity, depigmentation (dyschromia), and the appearance of telangiectasia. Later, hyperkeratosis (excessive keratinization) and sclerosis of the underlying subcutaneous fatty tissue may become apparent. After irradiation with hard X-ray or amma radiation, 6-9 months later. and later slowly progressive atrophy of muscle tissue and osteoporosis of bones are revealed. The most severe degree of muscle atrophy and bone growth retardation are observed in children. When treating malignant tumors, moist radioepidermitis is permissible only in small irradiation fields. A radiation ulcer can develop acutely in the coming days and weeks after intense single irradiation, subacutely after 6-10 weeks, and also several years after irradiation. The acute course is characterized by intense redness of the skin shortly after irradiation, accompanied by severe swelling, severe pain, and a disturbance in the general condition. On edematous skin with congestive hyperemia, large blisters often appear with hemorrhagic cloudy contents. Upon rejection of the epidermis, a necrotic surface is exposed, covered with a permanent plaque, in the center of which an ulcer forms. Over a long period of time, necrotic tissue is rejected, flaccid and unstable granulations form, and the ulcer undergoes epithelization. Often healing does not occur. Subacutely developing radiation ulcer is often the outcome of long-term wet epidermitis. In the tissues surrounding the ulcer within the irradiated field, pronounced radiation atrophy develops over the next few months. Late radiation ulcer usually develops against the background of sharply atrophied tissue at the site of irradiation. The formation of an ulcer occurs according to the type of acute radiation necrosis of tissue in the area of the entire irradiation field, involving not only the skin, but also the underlying tissues, subcutaneous tissue, muscles, and bones. In some cases, superficial excoriation (abrasion) appears on atrophied skin, which gradually deepens and increases in size, turning into a deep ulcer. Radiation skin atrophy and radiation ulcer often result in the development of radiation cancer. The result of radiation exposure to the skin and subcutaneous fatty tissue is often indurative tissue edema. Indurative edema develops as a result of damage not only to blood vessels, but also to lymphatic vessels, which leads to impaired lymph outflow, swelling and sclerosis of the skin and subcutaneous tissue. The skin and subcutaneous tissue of the irradiated field gradually become dense, rise above the level of normal skin, and when pressed, a pit remains. The skin is hyperpigmented, covered with telangiectasia, or acquires a reddish-bluish tint and becomes painful. Under the influence of trauma or for no apparent reason, skin necrosis may occur in the area of inductive edema, leading to the formation of deep radiation ulcers.
Erythema does not require special treatment; All you need is protection from any type of skin irritation: solar insolation, thermal, chemical and mechanical effects, washing, especially with soap. All of these irritants contribute to an increase in the degree of damage. It is allowed to lubricate reddened skin surfaces with indifferent fat, oils, and prednisolone ointment. Wet epidermitis is treated openly, without a bandage. The wet surface is treated daily or every other day with an alcohol solution of gentian violet. If necessary, apply dressings with aloe liniment, tesan emulsion, sea buckthorn oil, and fish oil. Epithelization ends after 1 - 2 weeks. Treatment of a radiation ulcer consists of radical surgical removal of the ulcer and surrounding tissues altered by radiation exposure. Non-radical intervention, i.e. leaving part of the irradiated tissue, leads to divergence of the sutures and the formation of an initially non-healing defect, which later turns into an ulcer again. After excision of small ulcers, it is possible to apply sutures without additional plastic surgery. For large ulcers, the operation ends with plastic surgery using flaps from surrounding tissues or Filatov flaps. Before the operation, long-term preparation is necessary, which consists of fighting the infection, for which antibiotics are used; to cleanse the ulcer from necrotic tissue, use a 5-10% solution of dibunol in linethol, peloidin, vinylin (Shostakovsky balm); To stimulate the formation of granulations, metacil ointment, fish oil, linol, and aloe liniment are used. To improve blood supply to the tissues surrounding the ulcer and increase its mobility in relation to the underlying tissues, as well as improve nerve trophism, a circular novocaine blockade with a 0.25% solution is used.
Share link:
Radiation necrosis: diagnosis
The main methods for diagnosing radiation necrosis are neuroimaging and histological examination of tissue from “suspicious” areas.
MRI is the most common method for diagnosing radiation necrosis. However, it should be noted that images of LN obtained using this method very often resemble the picture of tumor recurrence - with increased contrast in the affected area and edema located along the periphery of the pathological focus.
As a result, due to the low predictive value of conventional MRI, more modern diagnostic methods have become more important:
- magnetic resonance spectroscopy;
- perfusion MRI;
- positron emission tomography (PET).
Diagnosis of radiation necrosis using the first of these methods (magnetic resonance spectroscopy) is based on the fact that a viable tumor has an intact vascular system, as a result of which the volume of blood flow in it is greater compared to necrotic tissue.
For differential diagnosis in this case, the indicator of relative cerebral blood volume obtained using dynamic contrast MRI is used. For tumors, this figure is higher compared to radiation necrosis.
Assessing the metabolite composition of a metastatic brain tumor using magnetic resonance spectroscopy is another valuable method for differential diagnosis. Elevated choline/creatinine and choline/N-acetylaspartate ratios may indicate tumor recurrence rather than radiation necrosis.
Fluorotyrosine PET can also be considered a promising direction in the differential diagnosis of radiation necrosis. Some authors report a sensitivity of 100% and a specificity of 93% for the diagnosis of LN.
As for the use of histological examination to diagnose radiation necrosis, it must be emphasized that the volume of the biopsy specimen must be such as to be able to completely exclude tumor recurrence and at the same time not cause a clinically significant neurological deficit. When taking a biopsy, one should avoid violating the integrity of brain structures located deep in the central part of the thalamus, the motor area of the cerebral cortex, the occipital region and speech centers.
Typically, tissue samples affected by necrosis do not show a predominance of malignant cells. However, irradiated tumor tissue may contain necrotic areas, which does not always indicate radiation necrosis.
In radiation necrosis, examination of a biopsy specimen may reveal thickening of blood vessels with endothelial proliferation and/or hyalinization with fibrosis and moderate infiltration of lymphocytes and macrophages.
Radiation necrosis: treatment
For effective treatment of radiation necrosis, a correct diagnosis is of paramount importance, since an erroneous diagnosis of tumor recurrence and subsequent antitumor treatment can lead to undesirable severe consequences.
For patients without symptoms of radiation necrosis in whom a necrotic mass is detected by MRI during follow-up, a wait-and-see approach may be chosen. If the choice of treatment strategy does not depend on whether the patient has radiation necrosis or recurrent glioma, the patient's condition should be monitored using serial MRI.
However, treatment tactics should be different in the presence of symptoms such as space-occupying syndrome in the cranial cavity, increased intracranial pressure, and neurological disorders. In such cases, it is possible to use the following treatment methods (separately or in combination):
- administration of corticosteroids;
- if steroids are ineffective, the use of bevacizumab (a monoclonal antibody that selectively binds to and neutralizes biologically active vascular endothelial growth factor (VEGF) may be considered);
- hyperbaric oxygen therapy (oxygen supply under pressure of 2-3 atm. Per course of treatment: 20-30 sessions lasting 90-120 minutes each).
Surgical treatment of radiation necrosis has its advantages and disadvantages. Removal of the necrotic mass helps to quickly reduce increased intracranial pressure and restore neurological functions. However, such an operation is associated with a risk of serious complications and is performed mainly on patients in whom conservative treatment has failed.
Risk factors for radiation necrosis
To date, several risk factors have been identified that contribute to the development of radiation necrosis. According to leading experts in the field of neuro-oncology, despite the fact that these risk factors were mainly found in patients with arteriovenous malformations who were prescribed radiosurgery treatment, as well as patients with glioma, they can be extrapolated to all patients with brain metastases :
- tumor volume and absorbed dose;
- the use of chemotherapy along with radiotherapy;
- tumor localization (the risk of developing LN is maximum when irradiating the frontal cortex and is significantly lower when irradiating the brain stem region);
- histological features of the tumor.
It has also been established that radiotherapy for some metastatic brain tumors is associated with an increased risk of developing radiation necrosis, depending on the form of the primary tumor. An increase in the incidence of LN development is observed with radiotherapy of brain metastases in the following tumors:
- kidney carcinoma;
- lung adenocarcinoma;
- skin melanoma with BRAF V600 oncogene mutation;
- HER2-positive breast cancer.
In addition, increased individual sensitivity to the effects of ionizing radiation also contributes to the development of radiation necrosis.
Source: Radiation Necrosis
The effect of radiation therapy on hard dental tissues in patients with oropharyngeal cancer
E. F. Dmitrieva
postgraduate student of the Department of Orthopedic Dentistry and Orthodontics of the Federal State Budgetary Educational Institution of Higher Education "Southern State Medical University" of the Ministry of Health of the Russian Federation
N. S. Nurieva
Doctor of Medicine, Professor of the Department of Orthopedic Dentistry and Orthodontics, State Budgetary Educational Institution of Higher Professional Education "Southern State Medical University" of the Ministry of Health of Russia (Chelyabinsk)
Introduction
Cancer incidence in humans has gradually increased over the last century. The number of diseases with malignant tumors of the head and neck is also steadily increasing, reaching 20-25% of all malignant tumors in most regions of Russia. In particular, tumors of the oropharyngeal region account for 5.1% of all tumors [3].
The modern treatment strategy for patients with squamous cell carcinoma of the head and neck organs includes the use of surgery, radiation therapy, chemotherapy and targeted therapy, as well as various combination treatment options. Among various treatment methods in clinical oncology, radiation therapy occupies one of the leading places. According to WHO, 70-75% of cancer patients require radiation therapy.
Despite the latest advances in oncology, at present, after combined and radiation treatment of malignant neoplasms of the maxillofacial area, it is impossible to avoid complications associated with ionizing radiation. In particular, a side effect of radiation therapy is intense tooth decay. Damage to the hard tissues of teeth is associated both with the direct effect of radiant energy on them, and with the subsequent immunosuppressive state, disruption of mineral and protein metabolism, the amount and composition of saliva, and the functional state of the most important physiological systems of the body [4].
Purpose of the study
To study the features of the clinical picture of radiation-induced dental caries depending on the time elapsed after completion of radiation treatment of tumors of the oropharyngeal zone, which allows diagnosing the disease in the early stages.
Material and research methods
This work is based on the experience of clinical observation of 60 patients with stage II-IV malignant neoplasms of the oropharyngeal zone aged from 22 to 75 years. In the vast majority of cases, the histological conclusion is squamous cell carcinoma (in 97% of cases), in 3% of cases carcinoma is identified. The examined patients received a SPLIT course of EBRT: in 2 stages, SOD = 66–70 isoGy is applied to the tumor, to the l/nodes of the neck SOD = 44–50 isoGy, to metastases in the l/nodes of the neck SOD = 60–70 isoGy, to foci of lymphadenopathy more than 2.0 cm SOD = 60 isoGr. All patients completed the course of radiation therapy. Follow-up was carried out at 1, 6, 12 and 24 months after completion of radiotherapy. None of the patients received supportive dental therapy during the treatment phases and after discharge. Radiation caries was diagnosed during examination in 100% of cases two years after completion of specialized treatment (Fig. 1, 2).
We divided the changes in irradiated teeth as follows.
Radiation caries of the 1st degree: loss of shine, transparency of the enamel, the appearance of chalky spots as a manifestation of demineralization of the surface layer of enamel. However, there are no signs of destruction or formation of enamel defects. During probing, cavities are not detected. In the area of the neck of the teeth, a stuck probe is detected at the enamel-cement junction. Patients usually do not complain of spontaneous pain or pain from temperature stimuli. The temperature test (cold) is positive.
Radiation caries of the 2nd degree: the progression of the carious process is mainly observed in the cervical region. Characterized by destruction of areas of enamel, cavities with undermined edges. Decay does not affect dentin. Changes in color can range from brown to black. Increased abrasion of the enamel on the cutting edge and fragility and chipping of the enamel may be observed. Patients usually do not complain of spontaneous pain or pain from temperature stimuli. The temperature test (cold) is either slightly positive or negative.
Rice. 1. Patient V., 63 years old. Radiation caries. Rice. 2. Patient U., 68 years old. Radiation caries.
Radiation caries of the 3rd degree: complete destruction of enamel, disintegration of the enamel-dentin border. Dentin is softened upon probing. Positive percussion. Without spontaneous opening of the pulp. Patients usually do not complain of spontaneous pain or pain from temperature stimuli. Thermal test is negative. The absence of pain is based on the loss of vitality and sensitivity of the pulp.
Radiation caries of the 4th degree: complete destruction of the tooth crown, the pulp chamber is opened, the dentin is softened, dark in color. There was either no pain or periodontal pain.
The table shows the timing of the occurrence of radiation caries.
Results and discussion
Radiation dental caries (c. dentis radialis) is a generalized dental caries that develops as a complication of X-ray or radiotherapy of the maxillofacial area; occurs with pigmentation and softening of the surface layers and the formation of deep cervical cavities.
The pathogenesis of radiation damage to teeth is still not fully understood. Thus, data on vascular, morphological and degenerative disorders in the pulp that precede damage to the hard tissues of teeth are discussed [1]. In the oral cavity, during radiation therapy of the maxillofacial area, a cariogenic situation is created due to the development of radio-induced xerostomia, disruption of the oral microflora, difficulty in oral hygiene, decreased self-cleaning of the oral cavity and a general decrease in immunity. Despite the large number of studies on the etiology of radio-induced caries, there are still contradictions in opinions about the root cause of its occurrence: direct exposure to radiation or long-term indirect exposure to adverse factors that arise in the oral cavity after irradiation, the main of which is radio-induced xerostomia.
Until now, there has been no consensus on the nature of radiation damage to teeth. Some authors consider such lesions as non-carious, others - as an acute carious process.
Clinical manifestations of the consequences of radiation exposure can be very different and depend on the received radiation dose and the type of ionizing radiation [2]. Usually after 3-6 months. After radiation exposure, tooth enamel loses its characteristic shine and becomes dull and grayish-faded in color. Fragility and abrasion of the chewing and vestibular surfaces of the teeth are noted. Against this background, areas of necrosis appear, initially local, and then as a circular lesion of the teeth. These lesions are usually dark in color, filled with loose necrotic mass, and painless.
The absence of a pain symptom is a characteristic feature of radiation injury, indicating suppression of odontoblast function. Gradually, areas of necrosis expand and cover a significant part of the tooth. Removal of necrotic masses from the lesion is usually painless and therefore requires special care. If radical interventions are not used, after 1-2 years more than 96% of teeth will be affected [7].
Table No. 1. Timing of occurrence of radiation caries in patients receiving radiation treatment for tumors of the oropharyngeal zone, %
| Clinical manifestations | 0 - 1 month | 1 - 6 months | 6 - 12 months | 12 - 24 months |
| Radiation caries 1st degree | — | 58 % | 8 % | — |
| Radiation caries 2nd degree | — | 8 % | 58 % | 16 % |
| Radiation caries 3rd degree | — | — | 33 % | 17 % |
| Radiation caries 4th degree | — | — | — | 67 % |
Few research works are devoted to the problem of prevention and dental treatment of this pathological condition. Prevention of radiation caries includes a dental examination of patients before oncological treatment with mandatory sanitation of the oral cavity [5], professional hygiene, removal of completely destroyed teeth and tooth roots, as well as teeth with III degree of mobility, filling of all carious cavities. After this, individual hygiene products are also selected for patients and protective individual orthopedic devices are made [6].
Prevention of radiation caries immediately after oncological treatment should be aimed at treating xerostomia, careful oral hygiene, reducing the consumption of carbohydrate foods, and remineralizing therapy.
Timely treatment of dental caries even at the earliest stages of development of the pathological process is an important task in this group of patients [5].
Conclusion
Thus, our research allows us to draw the following conclusions. Radiation caries that occurs after radiation treatment of patients with cancer of the oropharyngeal zone is detected in 100% of cases with a radiation dose of up to 70 Gy, causing damage to the hard tissues of teeth from 6 to 24 months. The described clinical picture of radiation dental caries, depending on the time elapsed after completion of radiation treatment, makes it possible to diagnose and treat radiation caries in the early stages, which improves the quality of life of patients.