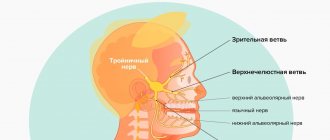
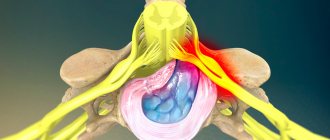
Dental implants solve a lot of problems in complex prosthetics, when there are difficulties in restoring acceptable function. When installing implants in the lower jaw, there is a significant risk of damage to any of the peripheral branches (inferior alveolar, mental, lingual nerve) of the mandibular part of the trigeminal nerve.
Although sensory impairment is a known and expected risk of some dental (medical and surgical) procedures, despite the highest quality care provided by the most qualified professionals, neurological complications are now the second most common cause of lawsuits against dentists in the United States.
Impaired sensitivity of oral structures is of great importance from a psychological and functional point of view. Anesthesia, soreness, or hypersensitivity may occur in the lips, cheeks, teeth, gums, or tongue. Drooling, choking on pieces of food or drinks, biting the lips or tongue, difficulty performing daily tasks such as shaving, applying makeup, talking, chewing, swallowing, kissing, smoking are a consequence of damage to the mandibular nerve and certainly cause extreme discomfort to the patient. especially if the patient was not warned about such complications in advance, and also if these problems are not completely resolved.
Nerve injuries do not always resolve on their own. However, in some patients they can be successfully treated using microsurgical techniques, provided they are performed promptly. In other patients, symptoms of nerve damage can be effectively managed with appropriate non-surgical therapy.
Causes of nerve damage
Injuries to the inferior alveolar, mental or lingual nerves result from compression, crushing, stretching, partial or complete rupture. In implantology practice, nerve rupture can occur when making an incision in the mucosa or when drilling into the bone to prepare an osteotomy hole for the purpose of inserting an implant. Nerve stretch occurs during prolonged retraction of the mucoperiosteal flap. Compression or crushing of the inferior alveolar nerve occurs as a result of the installation of a long implant. Injection of a local anesthetic may cause nerve damage through direct needle trauma or, more plausibly, through rupture of perineural or endonerval blood vessels with subsequent formation of scar tissue adhesions.
Results and its discussion
Performing RB according to generally accepted recommendations was effective in 88.9% with periorbital access to the maxillary nerve, in 71.4% with subzygomatic access to the maxillary nerve, and in 70.6% with blockade with subzygomatic access to the mandibular nerve.
(Table No. 1) Higher frequency of successful blockades according to V.F. Voino-Yasenetsky (p<0.05 compared with subzygomatic blockades) was probably associated with the implementation technique, which is based on achieving paresthesia during neurostimulation with a decrease in current intensity to 0.3 mA. Table No. 1. Failure rate depending on the type of regional block and the achievement of paresthesia
| Type of regional block | Study group | Paresthesia | Failure rate (%) |
| Periorbital maxillary nerve block | 1 | + | 11,1 |
| Subzygomatic block of the maxillary nerve | 2 | — | 28,6* |
| 4 | + | 9,8 | |
| Subzygomatic block of the mandibular nerve | 3 | — | 29,4* |
| 5 | + | 11,8 |
*p<0.05 - compared with groups with paresthesia
The high failure rate of trunk blockade of the maxillary nerve using the subzygomatic approach is directly related to incorrect interpretation of the motor response to neurostimulation. It must be remembered that the maxillary nerve is a purely sensory nerve and does not contain motor fibers. Therefore, the appearance of a motor response is associated with direct stimulation of the masticatory muscle, and when the needle is inserted into the pterygopalatine fossa, with stimulation of the lateral or medial pterygopalatine muscles [9].
Thus, the presence of a motor response should not be perceived as effective neuroimaging of the maxillary nerve and the injection of local anesthetic, in this case, will be carried out into the thickness of the muscle. In turn, intramuscular injection of a local anesthetic will prevent its spread along the pterygopalatine fossa and diffusion to the branches of the maxillary nerve.
High failure rate with mandibular nerve block according to S.N. Weissblatt, even when a muscle response is achieved, is associated with the anatomical features of the origin of sensory and motor fibers. Sensory fibers begin to separate from the trunk of the mandibular nerve immediately after its exit from the foramen ovale [10]. Additionally, unlike regional limb blocks, where the effectiveness of neurostimulation in the proximal region is assessed by the muscle response in the distal region, neurostimulation of the mandibular nerve leads to muscle contraction in the same area. In turn, such a contraction of the masticatory muscles in response to nerve stimulation can easily be confused with muscle contraction in response to direct muscle irritation.
Thus, to effectively block the mandibular nerve, it is necessary to bring the needle as close as possible to the foramen ovale and obtain paresthesia. The administration of a local anesthetic, focusing on muscle stimulation, can be associated either with direct stimulation of the masticatory muscle or with stimulation of purely motor branches or the mixed inferior alveolar nerve [9].
The change in tactics, which is associated with obtaining paresthesia, contributed to an increase in the number of successful subzygomatic RBs of the maxillary nerve to 90.2% and the mandibular nerve to 88.2%, respectively. However, about 10% of failures persisted with any type of blockade, which is likely due to anatomical features.
The use of 3D CT guidance avoids such failures. With the help of 3D-CT, it is possible to clearly identify the primary landmark for subzygomatic blocks (pterygopalatine process), with subsequent placement of the needle into the pterygopalatine fossa for maxillary nerve block or to the foramen ovale for mandibular nerve block. Moreover, in all successful cases (n=4), the needle was able to be brought to the required anatomical landmark after the second series of images, which made it possible to avoid an excessive increase in the X-ray dose.
3D-CT guidance on the primary landmark (outer plate of the pterygopalatine process) for trunk subzygomatic blockades of the maxillary and mandibular nerves according to S.N. Weisblat1. Zygomatic arch 2. Pterygopalatine process 3. Zygomatic bone 4. Auditory canal 5. Articular process of the mandible 6. Needle 7. Coronoid process of the mandible 8. Ramus of the mandible
3D-CT guidance for subzygomatic trunk blockade of the maxillary nerve according to S.N. Weisblath 1. Zygomatic arch 2. Needle 3. Lower jaw 4. Pterygopalatine space 5. Pterygopalatine process 6. Foramen ovale
3D-CT guidance for subzygomatic trunk blockade of the mandibular nerve according to S.N. Weisblath 1. Zygomatic arch 2. Needle 3. Ramus of the lower jaw 4. Pterygopalatine process 5. Foramen ovale
The combined use of neurostimulation and 3D-CT enabled safe needle advancement and avoided nerve damage. The needle was positioned in such a way as to obtain a feeling of paresthesia when the current intensity was reduced to 0.3 mA. In these cases, there was no need for the recommended limitation of needle advancement to the border at the level of the outer plate of the pterygopalatine process due to the risk of damage to the maxillary and mandibular nerves [3].
In addition, the use of 3D CT allows us to understand the reasons for failures when performing RB on the face. Positioning the tip of the needle at the border of the pterygopalatine process, as recommended in most RA guidelines, leads to lateralization of the block due to the inability of the local anesthetic to spread to the internal parts of the pterygopalatine fossa. Deviation of the needle tip from the foramen ovale caused the spread of local anesthetic in an area distant from the main trunk of the mandibular nerve and ineffective RB, despite a visible muscle response to neurostimulation with a current intensity of 0.3 mA.
Lateralization of the block when performing a trunk zygomatic blockade of the maxillary nerve according to S.N. Weisblath 1. Needle 2. Zygomatic arch 3. Zygomatic bone 4. Effective block area 5. Mandibular notch 6. Pterygopalatine space 7. Pterygopalatine process
Deviation of the needle from the foramen ovale as a cause of unsuccessful stem zygomatic blockade of the mandibular nerve according to S.N. Weisblath 1. Needle 2. Zygomatic arch 3. Foramen ovale 4. Mastoid process 5. Pterygopalatine process
Thus, the combined use of 3D-CT guidance and neurostimulation represents a new highly effective method of neuroimaging for brainstem blocks of the maxillary and mandibular nerves.
Prevention methods
Careful planning and skilled execution of manipulations minimize the risk of nerve damage. Panoramic and periapical radiographs, supplemented by scanning (if indicated), make it possible to determine the height of the alveolar process above the nerve, the medial lateral and vertical localization of the canal of the mandibular nerve and the mental foramen. Careful soft tissue incisions help avoid direct contact with the mental and lingual nerves, and gentle retraction of the flap minimizes indirect stretch of the nerve. When preparing the osteotomy hole and installing the implant, it is necessary to avoid damage to the mandibular nerve canal.
In the absence of sufficient height of the alveolar process to install an implant without the risk of damage to the canal of the mandibular nerve, lateralization of the mandibular nerve is indicated. Implants can be placed during this procedure (Figure 1-3).
Figure 1. Nerve reduction. The inferior alveolar nerve prevents adequate implant placement.
Figure 2. Transoral reduction of the nerve and its lateral retraction.
Figure 3. The implants are installed in the desired position and at the required depth, and the nerve is repositioned.
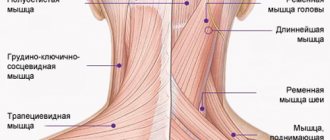
Branches of the trigeminal nerve: mandibular nerve
The mandibular nerve (n. mandibularis) is the third branch of the trigeminal nerve, is a mixed nerve and is formed by sensory nerve fibers coming from the trigeminal ganglion and motor fibers of the motor root (Fig. 1, 2). The thickness of the nerve trunk ranges from 3.5 to 7.5 mm, and the length of the extracranial part of the trunk is 0.5-2.0 cm. The nerve consists of 30-80 bundles of fibers, including from 50,000 to 120,000 myelinated nerve fibers.
Rice. 1. Mandibular nerve, left view. (Mandibular ramus removed):
1 - auriculotemporal nerve; 2 —
middle meningeal artery; 3 - superficial temporal artery; 4 - facial nerve; 5 - maxillary artery; 6—inferior alveolar nerve; 7 - mylohyoid nerve; 8—submandibular node; 9— internal carotid artery; 10—mental nerve; 11 - medial pterygoid muscle; 12—lingual nerve; 13— drum string; 14 - buccal nerve; 15 - nerve to the lateral pterygoid muscle; 16 - pterygopalatine node; 17 - infraorbital nerve; 18 - maxillary nerve; 19 - zygomaticofacial nerve; 20—nerve to the medial pterygoid muscle; 21 - mandibular nerve; 22 - chewing nerve; 23 - deep temporal nerves; 24 - zygomaticotemporal nerve
Rice. 2. Mandibular nerve, view from the medial side:
1—motor root; 2—sensitive root; 3—greater petrosal nerve; 4— lesser petrosal nerve; 5—nerve to the tensor tympani muscle; 6, 12— drum string; 7—auriculotemporal nerve; 8—inferior alveolar nerve; 9—mylohyoid nerve; 10—lingual nerve; 11 - medial pterygoid nerve; 13 - ear node; 14 - nerve to the muscle that strains the velum palatine; 15 - mandibular nerve; 16 - maxillary nerve; 17 - optic nerve; 18 - trigeminal node
The mandibular nerve provides sensory innervation to the dura mater of the brain, skin of the lower lip, chin, lower part of the cheek, anterior part of the auricle and external auditory canal, part of the surface of the eardrum, mucous membrane of the cheek, floor of the mouth and anterior two-thirds of the tongue, teeth of the lower jaw , as well as motor innervation of all masticatory muscles, the mylohyoid muscle, the anterior belly of the digastric muscle and the muscles that strain the tympanic membrane and the velum palatine.
From the cranial cavity, the mandibular nerve exits through the foramen ovale and enters the infratemporal fossa, where it divides near the exit site into a number of branches. Branching of the mandibular nerve is possible either according to the scattered type (more often with dolichocephaly) - the nerve breaks up into many branches (8-11), or according to the main type (more often with brachycephaly) with branching into a small number of trunks (4-5), each of which is common to several nerves.
Three nodes of the autonomic nervous system are associated with the branches of the mandibular nerve: the ear (ganglion oticum); submandibular (ganglion submandibulare); sublingual (ganglion sublinguale). From the nodes postganglionic parasympathetic secretory fibers go to the salivary glands.
The mandibular nerve gives off a number of branches.
1. The meningeal branch (r. meningeus) passes through the foramen spinosum along with the middle meningeal artery into the cranial cavity, where it branches in the dura mater.
2. The masticatory nerve (n. massetericus), predominantly motor, often (especially in the main form of branching of the mandibular nerve) has a common origin with other nerves of the masticatory muscles. It passes outward over the upper edge of the lateral pterygoid muscle, then through the notch of the mandible and is embedded in the masseter muscle. Before entering the muscle, it sends a thin branch to the temporomandibular joint, providing its sensitive innervation.
3. Deep temporal nerves (n. temporales profundi), motor, pass along the outer base of the skull outward, bend around the infratemporal crest and enter the temporal muscle from its inner surface in the anterior (n. temporalis profundus anterior) and posterior (n. temporalis profundus posterior ) departments.
4. The lateral pterygoid nerve (p. pterygoideus lateralis), motor, usually leaves a common trunk with the buccal nerve, approaches the muscle of the same name, in which it branches.
5. Medial pterygoid nerve (n. pterygoideus medialis), mainly motor. It passes through the ear ganglion or is adjacent to its surface and follows forward and down to the inner surface of the muscle of the same name, into which it penetrates near its upper edge. In addition, near the ear node, it gives the nerve to the muscle that tenses the velum palatine (n. musculi tensoris veli palatine), the nerve of the muscle that tenses the tympanic membrane (n. musculi tensoris tympani), and the connecting branch to the node.
6. The buccal nerve (p. buccalis), sensitive, penetrates between the two heads of the lateral pterygoid muscle and runs along the inner surface of the temporal muscle, spreading further along with the buccal vessels along the outer surface of the buccal muscle to the corner of the mouth. On its way, it gives off thin branches that pierce the buccal muscle and innervate the mucous membrane of the cheek (to the gum of the 2nd premolar and 1st molar) and branches to the skin of the cheek and corner of the mouth. Forms a connecting branch with the branch of the facial nerve and with the ear ganglion.
7. The auriculotemporal nerve (p. auriculotemporalis), sensitive, starts from the posterior surface of the mandibular nerve with two roots covering the middle meningeal artery, which then connect into a common trunk. Receives from the ear ganglion a connecting branch containing parasympathetic fibers. Near the neck of the articular process of the lower jaw, the auriculotemporal nerve goes upward and through the parotid salivary gland enters the temporal region, where it branches into terminal branches - the superficial temporal (rr. temporales superficiales). Along its path, the auriculotemporal nerve gives off the following branches:
1) articular (rr. articulares), to the temporomandibular joint;
2) parotid (rr. parotidei), to the parotid salivary gland. These branches contain, in addition to sensory ones, parasympathetic secretory fibers from the ear ganglion;
3) the nerve of the external auditory canal (n. meatus acustuci externi), to the skin of the external auditory canal and the eardrum;
4) anterior auricular nerves (auriculares anteriores), to the skin of the anterior part of the auricle and the middle part of the temporal region.
8. Lingual nerve (p. lingualis), sensitive. It originates from the mandibular nerve near the foramen ovale and is located between the pterygoid muscles anterior to the inferior alveolar nerve. At the upper edge of the medial pterygoid muscle or slightly lower, the chorda tympani (chorda tympani), which is a continuation of the intermediate nerve, joins the nerve. As part of the chorda tympani, the lingual nerve includes secretory fibers that go to the submandibular and sublingual nerve ganglia, and taste fibers to the papillae of the tongue. Next, the lingual nerve passes between the inner surface of the lower jaw and the medial pterygoid muscle, above the submandibular salivary gland along the outer surface of the hyoglossus muscle to the lateral surface of the tongue. Between the hypoglossal and genioglossus muscles, the nerve splits into terminal lingual branches (rr. linguales).
Along the course of the nerve, connecting branches with the hypoglossal nerve and the chorda tympani are formed. In the oral cavity, the lingual nerve gives off the following branches:
1) branches to the isthmus of the pharynx (rr. isthmi faucium), innervating the mucous membrane of the pharynx and the posterior part of the floor of the mouth;
2) the hypoglossal nerve (n. sublingualis) departs from the lingual nerve at the posterior edge of the hypoglossal node in the form of a thin connecting branch and spreads forward along the lateral surface of the sublingual salivary gland. Innervates the mucous membrane of the floor of the mouth, gums and sublingual salivary gland;
3) lingual branches (rr. linguales) pass along with the deep arteries and veins of the tongue through the muscles of the tongue forward and end in the mucous membrane of the apex of the tongue and its body to the border line. As part of the lingual branches, taste fibers pass to the papillae of the tongue, passing from the chorda tympani.
9. Lower alveolar nerve (n. alveolaris inferior), mixed. This is the largest branch of the mandibular nerve. Its trunk lies between the pterygoid muscles behind and lateral to the lingual nerve, between the mandible and the sphenomandibular ligament. The nerve enters, together with the vessels of the same name, into the mandibular canal, where it gives off multiple branches that anastomose with each other and form the lower dental plexus (plexus dentalis inferior) (in 15% of cases), or directly the lower dental and gingival branches. It leaves the canal through the mental foramen, dividing before exiting onto the mental nerve and incisive branch. Gives the following branches:
1) the mylohyoid nerve (n. mylohyoides) arises near the entrance of the inferior alveolar nerve into the mandibular foramen, is located in the groove of the same name in the branch of the mandible and goes to the mylohyoid muscle and the anterior belly of the digastric muscle;
2) the lower dental and gingival branches (rr. dentales et gingivales inferiors) originate from the lower alveolar nerve in the mandibular canal; innervate the gums, alveoli of the alveolar part of the jaw and teeth (premolars and molars);
3) the mental nerve (p. mentalis) is a continuation of the trunk of the lower alveolar nerve as it exits through the mental foramen from the canal of the mandible; here the nerve is divided fan-shaped into 4-8 branches, among which there are mental branches (rr. mentales), to the skin of the chin and lower labial (rr. labials inferiors), to the skin and mucous membrane of the lower lip.
The ear ganglion (ganglion oticum) is a rounded, flattened body with a diameter of 3-5 mm; located under the foramen ovale on the posteromedial surface of the mandibular nerve (Fig. 3, 4). The lesser petrosal nerve (from the glossopharyngeal) approaches it, bringing preganglionic parasympathetic fibers. A number of connecting branches extend from the node:
1) to the auriculotemporal nerve, which receives postganglionic parasympathetic secretory fibers, which then go as part of the parotid branches to the parotid salivary gland;
2) to the buccal nerve, through which postganglionic parasympathetic secretory fibers reach the small salivary glands of the oral cavity;
3) to the drum string;
4) to the pterygopalatine and trigeminal nodes.
Rice. 3. Autonomic nodes of the head, view from the medial side:
1 - nerve of the pterygoid canal; 2 - maxillary nerve; 3 - optic nerve; 4 - ciliary node; 5 - pterygopalatine node; 6 - greater and lesser palatine nerves; 7 - submandibular node; 8 - facial artery and nerve plexus; 9 - cervical sympathetic trunk; 10, 18 - internal carotid artery and nerve plexus; 11—superior cervical ganglion of the sympathetic trunk; 12 - internal carotid nerve; 13 - drum string; 14 - auriculotemporal nerve; 15 - lesser petrosal nerve; 16 - ear node; 17 - mandibular nerve; 19 - sensitive root of the trigeminal nerve; 20 - motor root of the trigeminal nerve; 21 - trigeminal node; 22 - greater petrosal nerve; 23 - deep petrosal nerve
Rice. 4. Ear node of an adult (preparations by A.G. Tsybulkin):
a — macromicroscopic specimen, stained with Schiff’s reagent, UV. x12: 1 - mandibular nerve in the foramen ovale (medial surface); 2— ear node; 3 - sensitive root of the ear node; 4 - connecting branches to the buccal nerve; 5 - additional ear nodes; 6 - connecting branches to the auriculotemporal nerve; 7 - middle meningeal artery; 8 - lesser petrosal nerve;
b — histotopogram, hematoxylin-eosin staining, UV. x10x7
The submandibular ganglion (ganglion submandibulare) (size 3.0-3.5 mm) is located under the trunk of the lingual nerve and is connected to it by nodal branches (rr. ganglionares) (Fig. 5, 6). Along these branches the preganglionic parasympathetic fibers of the chorda tympani go to the node and end there. The branches extending from the node innervate the submandibular and sublingual salivary glands.
Rice. 5. Submandibular ganglion, lateral view. (Most of the lower jaw has been removed):
1 - mandibular nerve; 2 - deep temporal nerves; 3 - buccal nerve; 4 _ lingual nerve; 5 - submandibular node; 6 - submandibular salivary gland; 7 - mylohyoid nerve; 8 - inferior alveolar nerve; 9 - drum string; 10 - auriculotemporal nerve
Rice. 6. Submandibular node (preparation by A.G. Tsybulkin):
1 - lingual nerve; 2 - nodal branches; 3 - submandibular node; 4 - glandular branches; 5 - submandibular salivary gland; 6 - branch of the submandibular node to the sublingual gland; 7 - submandibular duct
Sometimes (up to 30% of cases) a separate sublingual ganglion (ganglion sublingualis) is found.
Human anatomy S.S. Mikhailov, A.V. Chukbar, A.G. Tsybulkin
Published by Konstantin Mokanov
Classification of nerve injuries
Seddon described three types of nerve damage: neuropraxia, axonotmesis and neurotmesis. This classification is based on the relationship between the pathophysiology of nerve injury, the ability of the nerve to regenerate and clinical symptoms, which forms the basis for determining the prognosis of spontaneous recovery of sensitivity, indications and timing of surgery or other therapy.
Neuropraxia is a benign condition. There is temporary loss of sensation, but no anatomical damage to the nerve. Spontaneous recovery of sensitivity is possible within 4 weeks.
Axonotmesis is a more serious condition in which there is partial anatomical disruption of the integrity of the nerve and incomplete degeneration of the nerve distal to the injury. Initial symptoms of restoration of sensitivity do not appear until 6-8 weeks after the injury. Recovery may be incomplete (hypoesthesia) and is often accompanied by pain (dysesthesia).
Neurotmesis is a complete transection of a nerve or other complete disruption of its integrity with total degeneration of the portion of the nerve distal to the injury. There is little or no hope for spontaneous recovery. If the patient remains fully anesthetized for 3 months after injury, sensation is rarely recovered to a significant degree. Persistent and severe dysesthesia often develops. The progressive failure of the structures supporting the nerve and their replacement by scar tissue leads to the fact that, ultimately, 1 year after injury, even surgical intervention cannot restore nerve function in humans.
Treatment of trigeminal neuralgia in Saratov, how to treat trigeminal neuralgia in Russia
Sarklinik provides comprehensive treatment of trigeminal neuralgia in children and adults, treatment of inflammation of the trigeminal nerve (nervitis, first, second, third branches) in Saratov, Russia, which includes effective reflexology methods. You can cure trigeminal neuralgia in Saratov!
Sarklinik knows how to treat inflammation of the trigeminal nerve, how to cure neuralgia and trigeminal neuritis ! The following types of neuralgia are treated: neuralgia of the 1st (first) branch of the trigeminal nerve, neuralgia of the 2nd (second) branch of the trigeminal nerve, neuralgia of the 3rd (third) branch of the trigeminal nerve in children and adults. On the website sarclinic.ru you can see a doctor online for free. If you have neuropathy, nerve damage, pain, a cold, the trigeminal nerve hurts on the left, right, you have a cold, a cold, inflammation on the face, paralysis, paresis, you don’t know how to relieve the pain , then contact a doctor at Sarklinik.
Sign up for a consultation. There are contraindications. Specialist consultation is required.
Photo: Aniram | Dreamstime.com\Dreamstock.ru. The people depicted in the photo are models, do not suffer from the diseases described and/or all similarities are excluded.
Related posts:
Enuresis, bedwetting in children, treatment, child peeing at night
Poor attention: absent-mindedness, exhaustion, narrowing of scope, stiffness, distractibility
Facial nerve, facial nerve neuritis, treatment, inflammation, paresis, neuritis, neuropathy, facial nerve neuralgia
Obsessive neurosis: obsessive states, movements, treatment in Saratov, Russia
Neuralgia and neuritis, intercostal neuralgia: symptoms, treatment in Saratov
Comments ()
Grade
Documentation of nerve damage is necessary to evaluate sensory deficits, decide whether and when to perform surgery or other treatment, and for legal reasons.
The medical history should include the reason for the operation, the date of injury, and symptoms of sensory changes (if any).
A study is being conducted to assess the severity of sensitivity disorders, for which neurosensory tests are used (response to irritating stimuli, static electricity, vitalometer, etc.). Re-evaluation is performed every 4 weeks until sensitivity is acceptable or other surgery is required.
Indications for microsurgery
The indications for surgery to correct nerve damage are based on the author's own experience, which includes the observation of more than 1000 patients and the performance of 375 microsurgical procedures to correct nerve damage, 21 of which resulted from dental implants between 1987 and 1996. year. The author's opinion regarding indications for surgical interventions is shared by many specialists in the field of microsurgery.
- Open (visible) nerve damage should be repaired as soon as possible. Such injuries usually occur during operations to install implants.
- Closed (non-visualized) damage must be repaired in the following order:
- Anesthesia lasting more than 3 months is reversed by suturing or nerve grafting.
- Dysesthesia that is unacceptable to the patient and lasts more than 4 months is eliminated with open exploration of the nerve through external decompression, internal neurolysis, excision of the neuroma, suturing and nerve transplantation.
- Severe hypoesthesia, unacceptable to the patient and lasting more than 4 months, can be eliminated by removing or partially unscrewing the implant, as well as by open exploration of the nerve and performing the manipulations described above.
- In general, patients who do not return to normal sensation within 4 weeks of surgery should be referred to a microsurgery specialist who can monitor the patient and modify the treatment plan as needed.
Inflammation, trigeminal neuralgia symptoms
Trigeminal neuralgia is clinically manifested by short-term attacks of excruciating pain, most often in the area of the 2nd and 3rd branches of the trigeminal nerve. It is characterized by the presence of trigger zones on the skin and mucous membranes. Touching them provokes attacks of pain. In most cases an attack of pain is accompanied by severe local and general autonomic disorders, the following symptoms: facial hyperemia (redness of the face) and swelling on the affected side, lacrimation, rhinorrhea (discharge of watery mucous discharge from the nose), hypersalivation (salivation, increased secretion of the salivary glands), Possible increased blood pressure (BP), chills-like trembling, difficulty breathing.
Other treatments
In general, implant removal does not eliminate nerve damage when treating nerve damage early. Nerve injury most often occurs during incision, flap retraction, or during osteotomy hole preparation. Nerve compression by an implant, unless severe, rarely results in permanent nerve injury.
Moderate dysesthesia or a syndrome of long-term (more than 1 year) painful nerve injury can sometimes be successfully treated with non-surgical treatments. Antineuralgic drugs (carbemazepine, phenytoin, clonazepam, bacolofen) or antidepressants (amitriptyline, notriptyline, imipramine), topical applications (capsaicin) or local anesthetic components (mixeltin) taken orally help patients with severe dysesthesia for whom surgery is not indicated or did not help. Other therapies (eg, acupuncture, transcutaneous electrical nerve stimulation, psychological or psychiatric therapy, physical therapy) may play a role in treating the painful manifestations of nerve damage.
results
The prognosis for improvement or restoration of sensitivity after microsurgery depends on the age of the patient, the technical skills of the surgeon, and the length of the period between the fact of damage and surgical intervention aimed at eliminating its consequences.
From the author’s experience, in 80-90% of patients suffering from neurotmesis (usually expressed by anesthesia), operated on up to 6 months after the injury, it was possible to improve or restore sensitivity. Interventions performed more than 6 months after injury resulted in improvement in fewer patients. When operations were performed 1 year after the injury or later, the condition of less than 10% of patients improved. Patients who underwent surgery to correct dysesthesia after 9 months achieved improvement in 70% of cases, with worsening results as time to surgery increased.