Currently, more and more attention is being paid to how immunity affects various processes in our body. Disturbed immune responses discovered in new studies become the missing link in pathogenesis. This stimulates the search for new drugs and other preventive and therapeutic effects. In this text we will discuss the special immune status of the brain - its isolation from systemic immune processes. In addition, let's talk about disorders that violate the immune sovereignty of the central nervous system - autoimmune encephalitis.
Privileged body
The brain is an organ that is remarkable in many ways [1]. The infinite complexity of the device, its functionality and the connection between our lives and its state attract the attention of researchers to the brain. The relationship between the brain and the immune system of our body is also special: the brain is an immune-privileged organ. Immune reactions that easily develop in other tissues (liver cells, muscles, fatty tissue) rarely occur in the brain. Along with the brain, the thyroid gland, testicles and some eye tissues, in particular the cornea, found themselves in such a special relationship with the immune system.
In the minds of doctors in the second half of the twentieth century, the brain was a structure completely isolated from the immune processes of the rest of the body. At the beginning of the last century, Japanese scientists conducted experiments on implanting sarcoma cells (a malignant tumor of muscle tissue) into various organs of mice [2]. It turned out that when sarcoma cells are placed in brain tissue, the tumor actively develops, but when they are implanted under the skin of rodents or into muscle tissue, the tumor does not develop. The researchers suggested that the “center” (the brain) is isolated from the immune processes that successfully fought off the sarcoma “in the periphery” (other tissues). Further research only confirmed this conclusion. The brain, in the minds of scientists, has become a “clean zone” in which an immune response does not develop. In the 1950s, the term “immunological privilege” arose, coined by British scientists named Billingham and Boswell [3].
Overcoming barriers
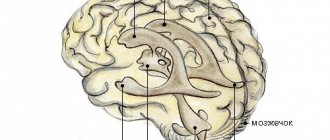
It has long been believed that the basis of the immune privilege of the brain is the presence of the blood-brain barrier (BBB). The BBB is a complex of cellular and extracellular structures that separate the blood flowing in the capillaries from the neurons of the brain parenchyma. The cells of the vascular walls, the basement membrane on which they lie, and astrocytes participate in the formation of the BBB. The immune privilege of parts of the central nervous system was in good agreement with the extent of the BBB within it. The brain parenchyma is reliably protected by the BBB, and inflammatory processes (encephalitis) rarely occur in it. The choroid plexus, which produces cerebrospinal fluid, and the membranes of the brain do not have such cover, and their inflammation (choroiditis, meningitis) is much more common (Fig. 1).
Figure 1. Structure of the central nervous system. The parts that are well protected by the BBB include the gray and white matter. Other components do not have this protection.
[2]
There are cases in which even the BBB does not save the nervous system, and inflammation processes occur in the brain. However, it is important to answer the question for yourself: do they proceed in the same way as in the rest of the body? Maybe the brain has a special way of immune response, and immune privilege has additional explanations? In vitro, an immune response in tissues can be induced by injecting bacterial lipopolysaccharide (LPS). It is a structural component of the cell wall of gram-negative bacteria that triggers a cascade of immune reactions in our body. When studying the reaction of different body tissues to the introduction of LPS, it turned out that an immune response in the skin and choroid plexuses of the central nervous system occurs to the same dose of LPS [4]. This is logical, because in neither case is there special protection in the form of the BBB. At the same time, to cause an immune reaction in the brain parenchyma similar to that occurring in the skin, a dose 100 times greater than the “skin” dose is required. In this case, compartmentalization of the immune response occurs - predominantly the white matter (the processes of neurons) is affected, and not the gray matter (their bodies). This experience points to another explanation for immune privilege: the weakness of our brain's own, “intrinsic” immune response. The brain parenchyma turns out to be extremely passive in relation to bacterial invasion: the brain does not seem to want to notice foreign components inside itself. Next we will try to figure out what is the reason for this immune “inattention”.
Weak link
If we are talking about the weakness of the local immune response, then we need to figure out what is “broken” in it. This may be impaired recognition with the normal ability of immune cells to attack antigens (weakness of the afferent link). Another option: normal antigen recognition with impaired ability to attack it (weakness of the efferent link). Studies have shown that the efferent part of the brain is preserved and functions normally [2]. Then efforts were focused on searching for disorders in the afferent link. Over time, it became clear that the problem was in recognizing the antigen and further working with it (Fig. 2).
Figure 2. The immune response occurs in several stages. The formation of a reaction to an antigen occurs in two ways: information about the antigen is delivered to local lymph nodes in “liquid” (the antigens themselves) and “solid” form (previously activated cellular elements of the immune system). In an immune reaction outside the brain, both pathways operate. When the brain's immune system reacts to an antigen, cellular elements do not reach the local (cervical) lymph nodes. It is one of the main components of a weak immune response in the brain.
[2]
You can read more about these processes in the article “Immunity: the fight against strangers and... our own” [5].
The brain has difficulty delivering activated immune cells to the cervical lymph nodes. Dendritic cells are responsible for working with antigens in the brain. In other organs, they recognize the antigen and then provide information about it to T and B lymphocytes in the lymph nodes. During inflammation in the brain, this process does not occur: dendritic cells do not migrate to the lymph nodes and do not present antigen. The immune response becomes localized and is regulated by dendritic cells in the brain parenchyma. If in other organs the cellular elements of immunity, after the presentation of the antigen, rush to the site of bacterial penetration [6], then with the development of inflammation in the brain parenchyma this does not happen. The brain has to rely on itself. A similar locality of the immune response is observed in the parenchyma, but not in the choroid plexuses and meninges.
Immigrants in the brain
If we continue to describe the special immune status of the brain in our body, then it is necessary to say a few words about microglia. Microglia are the main cell type responsible for the innate immune response in the brain (Fig. 3) [7]. Microglia have phagocytic activity, are able to recognize microorganisms and contribute to the development of inflammatory reactions in the brain parenchyma, that is, they behave not like a neuron, but a real immune cell [8]. This is explained by the fact that microglia in our brain are of hematopoietic origin [9]. The precursors of microglia in the prenatal period are the “brothers” of all other immune cells that find themselves in the central nervous system. They did not lose their immune functions and began to carry them out locally. It turns out that microglial cells can be called emigrants of the immune system.
Infarctions and necrosis of the brain (cerebellar hemispheres)
Infarctions and necrosis of the brain (cerebellar hemispheres)
It is possible to distinguish between infarctions (necrosis) of the cerebral hemispheres, cerebellum, basal ganglia, optic thalamus and brain stem. In this book, I do not consider ischemic strokes, which occur more often in children of several months and years of life, which have much in common with strokes in adults, which are differentiated into thrombotic (due to vascular pathology, thrombosis of the sinuses and veins, vasculitis, etc.), embolic (associated with heart pathology, paradoxical embolism, sepsis, placental infarctions, etc.), hemodynamic (with cardiomyopathies, narrowing of the great vessels, etc.) and metabolic (with diabetes mellitus, homocysteinuria, metabolic acidosis, Fabry's disease, Ley's disease, etc.). The work provides data on brain lesions mainly in newborns, which have their own specifics, have not been sufficiently studied and may be associated with birth trauma.
Cortical infarctions occur predominantly in full-term newborns. This is due to active differentiation, a high level of metabolism and better blood supply to the cortex than in premature infants.
The heart attacks in question have not been studied enough, and when describing individual cases, authors use different terms. Apparently, there are different types of heart attacks, which is associated both with the characteristics of their morphology and the causes that caused them. K. Courville identifies “cortical-subcortical softening” of the cerebral hemispheres, which is associated with microcirculation disorders, asphyxia during childbirth and vasomotor disorders of large arteries. According to A. Tovbin, hypoxia in full-term infants leads to stasis-thrombosis of the veins of the pia mater with the subsequent development of laminar necrosis and infarction of the subcortical white matter of the brain. Considering intracerebral hemorrhages, RL Friede notes the existence of hemorrhagic damage to the subcortical white matter and wedge-shaped hemorrhagic zones in the cortex, not associated with birth trauma, which, when examined microscopically, look like hemorrhagic infarctions. At the same time, he describes heart attacks that occur due to blockage of the arteries and thrombosis of the sinuses and veins, which other authors also discover (see below).
J. Larroche (1977) identifies hemorrhagic infarctions of the white matter, combined with cortical necrosis, the genesis of which is associated with arterial thrombosis, as well as hemorrhagic infarctions of the cortex and subcortical white matter, resulting from compression of the brain by a subdural or subarachnoid hematoma, thrombosis of the sinuses and veins. In addition, in the section on predominantly arterial ischemic lesions of the brain, he describes ischemic necrosis of the cortex, often combined with necrosis of various structures of the gray matter of the brain. This lesion is characterized by the following changes: cerebral edema, a clear boundary between the pale cortex and sharply stagnant subcortical white matter (the “ribbon” effect), more pronounced changes in the depth of the sulci. Microscopically, in areas of necrosis, a decrease in the number of nerve cells, shrinkage of neurons with pyknosis of the nuclei and acidophilia of the cytoplasm, karyorrhexis phenomena, proliferation of glia and capillaries are observed. Cortical necrosis may be laminar or patchy. Due to softening of the cortex and subcortical white matter, lace-like or honeycomb-like lesions, as well as cortical sclerosis and ulegyria, can develop. Ischemic necrosis of the cortex occurs in children born with asphyxia and during complicated labor (long second stage, application of obstetric forceps, prolapse of the umbilical cord, difficult extraction of the head during breech presentation, etc.). Similar lesions were observed in experiments on monkey fetuses when partial asphyxia was caused in them (with partial placental abruption, compression of the mother’s abdominal aorta, administration of oxytocin, induction of maternal hypotension, etc.). As a result, either the entire cerebral cortex is affected, or the process is limited to the paracentral or parietal areas.
J. Volpe identifies “parasagittal brain lesions,” which he previously called “watershed infarctions.” These lesions are found in full-term children and represent areas of bilateral symmetrical necrosis of the cortex and subcortical white matter in the border zones between the branches of the anterior, middle and posterior cerebral arteries, localized in the superomedial parts of the cerebral hemispheres. The author associates the pathogenesis of these ischemic (sometimes hemorrhagic) infarctions with hypoxia, arterial hypotension and disorders of autoregulation of cerebral circulation. Some researchers have observed necrosis of the cortex and underlying white matter in the parasagittal areas of the brain of newborn monkeys whose mothers suffered (in the experiment) arterial hypotension. In another study, sheep fetuses were exposed to hypovolemia and hypoxia. In this case, symmetrical hemorrhagic infarctions were observed predominantly in the parasagittal areas of the cortex and white matter, which also involved the basal ganglia and visual thalamus.
JI. Roerke calls infarctions of the cerebral hemispheres cerebral necrosis, believing that they are located in the border zones, between the branches of the main cerebral arteries. His descriptions of these lesions are generally consistent with the results of J. Larroche's study of cortical necrosis. At the same time, the author distinguishes between “chronic cortical necrosis”, which includes “cystic cortical infarctions”, “gyral sclerosis”, “microgyria” and other changes.
Infarctions of the cerebral hemispheres can be caused by blockage of the main branches of the cerebral arteries (most often the middle cerebral arteries). There are descriptions of only isolated cases of these lesions. Blockage of the arteries occurs with embolism, which may be associated with placental infarction, catheterization of blood vessels or the heart, or rupture of blood clots from the umbilical vein or ductus arteriosus. Morphologically, infarctions are often mixed and represent a combination of ischemic infarction of the cortex and hemorrhagic infarction of the underlying white matter. The consequences of heart attacks in surviving children are cystic encephalomalacia, unilateral or bilateral cystic cavities, porencephaly and hydrocephalus.
There are also separate descriptions of cerebral hemisphere infarctions that develop as a result of thrombosis of the sinuses and superficial veins of the brain. A feature of these lesions is that the infarctions are hemorrhagic in nature both in the cortex and in the subcortical white matter. Most often, blood clots are detected in the superior sagittal sinus, and in its middle part. Superficial cerebral vein thrombosis is usually secondary to sinus thrombosis. Infarctions are most often localized in the convexital areas of the cerebral hemispheres, involving the cortex and underlying white matter. The genesis of sinus and vein thrombosis is associated with birth trauma, hemocoagulation disorders, disseminated intravascular coagulation syndrome, infectious diseases, complications of puncture of the superior sagittal sinus, etc. The consequences of such infarctions are glial scars, cysts, lobar sclerosis and hydrocephalus.
I will give 3 observations of cerebral hemisphere infarctions in newborns. In the first case, in a 5-day-old newborn with disseminated intravascular coagulation syndrome, numerous blood clots were found in the superficial branches of all cerebral arteries of the two hemispheres of the brain. There were multiple diapedetic hemorrhages in the subcortical white matter, clearly demarcated from the pallid cortex and microscopically often located around thrombosed small vessels. The white matter was in a state of necrosis with widespread pyknosis and rhexis of gliocyte nuclei. Karyorrhexis and ischemic changes were detected in many nerve cells of the cortex. In the second case, in a newborn who died 36 hours later from a birth injury to the skull, hemorrhagic infarcts were located in various parts of the cortex, mainly in the depths of the convolutions of the two hemispheres of the brain (as well as in the visual thalamus). Microscopically, ischemic changes in many neurons were detected. Thrombi were detected in single branches of the superficial and basal veins. These infarctions, apparently, were caused not so much by venous thrombosis, but by disturbances of venous outflow during the period of protracted labor with the head standing in one plane for a long time. In the third case, in a full-term newborn with hemolytic disease, ischemic infarction of the cortex and underlying white matter was located under the subpial hematoma, which was probably caused.
Taking into account our own and literature data, infarctions should be differentiated into 3 types - white, hemorrhagic and mixed. White infarctions occur in newborns born in “asphyxia”, during complicated labor (long second stage, prolapse of the umbilical cord, application of forceps, difficulty removing the head during breech presentation, etc.) and with compression of the brain. Therefore, these lesions may be associated with birth trauma. They are characterized by: cerebral edema, a clear boundary between the pale cortex and sharply stagnant subcortical white matter (the so-called “ribbon effect”), more pronounced changes in the depth of the sulci. The brain has a characteristic appearance: the convolutions are sharply smoothed due to their close adherence to the inner surface of the bones of the roof of the skull, and the grooves are narrowed and do not contain visible cerebrospinal fluid; the brain is soft, fragile and easily damaged when removed from the cranial cavity. In some cases of severe lesions, the bark instead of white becomes light brown with fine grain. The parasagittal areas of the cortex are most susceptible to damage.
Microscopically, in areas of necrosis, a decrease in the number of nerve cells, shrinkage of neurons with pyknosis of the nuclei and acidophilia of the cytoplasm, karyorrhexis phenomena, proliferation of glia and capillaries are observed. Cortical necrosis may be laminar or patchy. Due to softening of the cortex and subcortical white matter, children may subsequently develop “lace-like” or “honeycomb” lesions, as well as cortical sclerosis
Mixed infarcts are a combination of white cortical infarction and hemorrhagic infarction of the underlying white matter. They may be caused by blockage (thrombosis, embolism) of the main branches of the cerebral arteries (usually the middle ones). Arterial embolism can be associated with placental infarctions, catheterization of blood vessels and the heart, and rupture of blood clots from the umbilical vein or ductus arteriosus.
Hemorrhagic infarctions occur when venous outflow is disrupted in cases of compression of the veins and sinuses during childbirth, as well as with thrombosis of the sinuses and superficial veins of the brain. Infarctions are localized primarily in areas of the convexital surfaces of the cerebral hemispheres.
The presented data on infarctions of the cortex and subcortical white matter in fetuses and newborns indicate insufficient knowledge of the etiology, pathogenesis and morphology of this group of cerebral hemodynamic disorders. Certain difficulties are also created due to terminological confusion arising from the presence of various terms that often denote the same processes.
Cerebellar infarctions occur both hemorrhagically and ischemically. The latter include, first of all, the so-called “leaf infarctions”, which often occur with subarachnoid hemorrhages of the cerebellar hemispheres, disrupting the nutrition of the underlying brain tissue. Cerebellar infarctions can be extensive, involving the entire hemisphere, or focal, localized in the cortex. They are often hemorrhagic in nature, located on the border between the blood supply zones of the superior and inferior cerebellar arteries. The pathogenesis of cerebellar infarctions is associated with compression of its arteries (for example, tentorium during childbirth), damage to the vertebral arteries, herniation of the tonsils into the foramen magnum and other reasons.
Infarctions of the basal ganglia, optic thalamus and brain stem. These lesions, which are pronounced and macroscopically visible, are very rare. With lesions of the trunk, they are most often found in the area of the base of the bridge, the lower tubercles of the quadrigeminal and the lower olives. Their pathogenesis is associated with severe circulatory complications due to shock, pulmonary heart failure, thrombosis of the basilar artery and other causes.
Autoimmune encephalitis
After talking about the special immune status of the brain, we will move on to the topic of autoimmune encephalitis - a group of diseases that are associated with damage to the membrane and intracellular structures of neurons by the body’s own immunity. In modern practice, autoimmune encephalitis is rarely diagnosed. This is explained by the fact that the first cases were described in detail only in 2005 [10]. It can be assumed that there are actually more cases of this disease than are recorded by specialists. Some patients with autoimmune encephalitis may be diagnosed with other disorders, such as schizophrenia or infectious encephalitis. This is due to the fact that doctors have little knowledge about this disease. Doctors diagnose only those diseases that they know. The more arsenal of diagnoses a doctor has in stock, the more accurate the diagnosis and the more correct the treatment.
Autoimmune encephalitis can be divided into two groups:
- diseases caused by activated T cells (something similar occurs in multiple sclerosis [11]);
- diseases that occur when antibodies act on intra- and extracellular components of a neuron, such as ion channels.
The first group of autoimmune encephalitis is characterized by cell damage by antibodies and activated T lymphocytes [12]. These encephalitis are more severe and require intensive therapeutic interventions, unlike representatives of the second group.
In the second group of encephalitis, neuronal damage is only “superficial” in nature. The effect of specific antibodies on surface-located structures leads to the “contraction” of receptors (in English this is called capping) and their subsequent internalization (capture) into the cytoplasm (Fig. 3) [13]. In addition, autoantibodies themselves can bind to receptors, blocking their work [14].
Figure 3. The changes that occur in receptors in autoimmune encephalitis are similar to the changes in synapses in myasthenia gravis. Normally (left side of the figure), the receptors are free and easily interact with the neurotransmitter acetylcholine. In case of illness, antibodies begin to affect the receptors, and their ability to bind to the neurotransmitter is impaired. The “clumped” acetylcholine receptors are gradually drawn into the cytoplasm, where they are destroyed.
www.memorangapp.com
Antibodies penetrate from the outside, from outside the BBB, or are produced by brain-infiltrated and activated B-lymphocytes [15]. In cases where antibodies are directed against intracellular structures, the attack on neurons is led by cytotoxic T lymphocytes. With the help of perforin and granzyme B, they damage the membrane of neurons, which leads to their death [16], [17]. The GEB, which we talked about above, in this light appears to be a reliable fortress wall protecting a quiet city that is no longer used to fighting. If a gap appears in the wall, the city will quickly fall: the nerve cells will be too sensitive to the effects of immune factors.
Looking for flags
Signs of autoimmune encephalitis are very varied, but three types of symptoms can be distinguished.
- Psychiatric symptoms: psychosis, aggressive actions, sexual disinhibition, panic attacks, obsessive actions, feelings of euphoria or fear.
- Motor symptoms: increased muscle tone and unevenness, muscle twitching and repetitive movements of the limbs.
- Seizures: generalized epileptic seizures, status epilepticus.
The initial manifestations of autoimmune encephalitis may be symptoms of a mental disorder: memory impairment, hallucinations or the appearance of delusional ideas. For example, with autoimmune damage to glutamate NMDA receptors, 80% of patients showed symptoms of mental illness, and more than 60% were initially hospitalized in psychiatric departments [18], [19]. Psychiatrists must be able to identify patients with autoimmune encephalitis or at least suspect this disease in order to promptly send the patient for appropriate examinations and consultations with other doctors.
Among the variety of symptoms that can be caused by different reasons, it is easy to get lost. The doctor needs at least rough guidelines to suspect the diagnosis of autoimmune encephalitis. As a result of the analysis of many cases of the disease, signs were identified that most likely indicate this diagnosis - the so-called “red flags” of autoimmune encephalitis [20]. These include:
- Lymphocytic pleocytosis or the appearance of oligoclonal bands on CSF electrophoresis.
- Epileptic seizures.
- Faciobrachial dystonic seizures.
- Suspicion of neuroleptic malignant syndrome.
- Abnormalities on MRI.
- EEG abnormalities.
In addition, the researchers also identified “yellow flags” - signs that should alert the doctor and make him think about a possible diagnosis of autoimmune encephalitis [20]. Yellow flags for autoimmune encephalitis are:
- Reduced level of consciousness.
- Violation of posture and movements.
- Instability of the autonomic nervous system.
- Focal neurological symptoms.
- Speech disorders (aphasia and dysarthria).
- Rapid progression of psychosis, despite treatment.
- Hyponatremia.
- Catatonia.
- Headache.
- The presence of other autoimmune diseases, including thyroiditis.
If a doctor sees symptoms from the group of “red flags” in a patient, the study authors recommend testing for specific autoantibodies for the timely diagnosis of autoimmune encephalitis. If a patient has symptoms from the list of “yellow flags,” the doctor should suspect the possibility of such a diagnosis and examine the patient more carefully to look for more reliable signs of autoimmune encephalitis.
Additional Arguments
In addition to the combination of previously described symptoms, the diagnosis of autoimmune encephalitis must be confirmed by laboratory tests and other diagnostic procedures [21].
For example, you can test for antibodies to specific receptors. In autoimmune encephalitis that affects glutamate NMDA receptors, an increase in the titer of antibodies to them can be detected. Interestingly, in encephalitis the amount of immunoglobulins of class G increases, and in schizophrenia - classes A and M [22]. Currently, a correspondence has been established between antibodies that affect specific neuron structures and the symptoms of encephalitis (Table 1). Magnetic resonance imaging (MRI) may show changes in brain structure, and electroencephalography (EEG) may show abnormal brain function in the case of autoimmune encephalitis. Table 1. Correspondence of symptoms and autoantibodies in autoimmune encephalitis [20]
| Structure to which an antibody is produced | Psychiatric symptoms | Other symptoms | Typical patient |
| NMDA receptor | Psychosis, schizophrenia-like disorders, catatonia, aggression | Epileptic seizures, dyskinesia, autonomic instability, speech and consciousness disorders | Young women, common association with ovarian teratoma |
| Caspr2 | Insomnia, panic attacks, depression, schizophrenia-like disorders | Morvan's syndrome, neuromyotonia, muscle spasms, fasciculations | Middle-aged and older patients, possible connection with thymoma |
| LGI1 | Amnesia and other memory impairments, confusion, depression | Limbic encephalitis, faciobrachial dystonic seizures, hyponatremia | Middle-aged and older patients, male to female ratio 2:1, possible association with thymoma |
| Glycine receptor | Behavioral changes, schizophrenia-like syndrome | Tight muscle syndrome, progressive encephalomyelitis with rigidity, myoclonus, hyperekplexia | Middle-aged and older patients, possible association with thymoma and lymphoma |
| Synaptic antigens (GAD) | Schizophrenia-like syndrome, autism, attention deficit hyperactivity disorder | Limbic encephalitis, stiff muscle syndrome, seizures, brainstem dysfunction, ataxia | Middle-aged and older patients, possible association with small cell lung cancer |
| Onconeural antigens (Yo, Hu, CV2, Ri, Ma2) | Behavioral disorders | Limbic encephalitis, cerebellar degeneration, sensory neuropathy | Elderly patients, often with malignant tumors |
In some cases, autoimmune damage to the central nervous system is caused by the development of paraneoplastic syndrome, which may accompany the appearance of a malignant tumor in the body. In paraneoplastic syndrome, the tumor can begin to independently produce hormones and hormone-like substances, disrupting the regulation of various processes in the body. In addition, paraneoplastic syndrome can manifest itself in the form of an autoimmune disease, for example, autoimmune encephalitis.
The pathogenesis of autoimmune encephalitis within the paraneoplastic syndrome is as follows. In a malignant tumor of a certain tissue, genes that are typical for it are expressed, as well as genes that are usually “silent” in it. Among the “silent” genes there may be those that are normally expressed only in the brain, under the protection of the BBB. Therefore, the resulting proteins are called cancer-neuronal antigens. During the maturation of the immune system, it becomes familiar with almost all the proteins of the body, but these proteins are hidden from it and, accordingly, are perceived as foreign. From the point of view of our immunity, there is no difference between them and bacterial antigens. The tumor begins to produce cancer-neuronal antigens, and the immune system recognizes them and produces autoantibodies specific to them. Through minimal gaps in the BBB, autoantibodies penetrate the central nervous system and begin to attack neurons [14].
For this reason, when diagnosing autoimmune encephalitis, special attention is paid to searching for a tumor in the patient’s body. Removal of the tumor in cases of paraneoplastic origin of autoimmune encephalitis will lead to a significant improvement in the patient's condition. While the tumor is in the human body, antibodies continue to be produced, and the patient’s condition worsens.
A blow to the immune system
Steroids and immunoglobulins administered intravenously are used as first-line drugs for the treatment of autoimmune encephalitis. Steroids have a powerful anti-inflammatory effect. They are capable of suppressing immune responses at a variety of levels. The use of steroids leads to a decrease in the synthesis of inflammatory mediators and stabilization of lysosome membranes that secrete inflammatory factors. In addition, under the influence of steroid hormones, the migration of monocytes decreases. Plasmapheresis, which cleanses the blood of “extra” antibodies, can also help.
If first-line therapy does not work, then switch to the use of rituximab and cyclophosphamide. Rituximab is a monoclonal antibody against the CD20 receptor, which is found on the surface of B lymphocytes [23]. B lymphocytes produce a variety of antibodies, including autoantibodies against nerve cells, and their destruction can lead to improvement. The CD20 receptor appears on the surface of normal B-lymphocytes and B-lymphocytes that have undergone the process of malignancy (malignancy). Due to this property, rituximab is effective in autoimmune diseases and B-cell lymphomas. The drug binds to the CD20 receptor on the surface of the lymphocyte and enables other components of the immune system to destroy the cell.
Cyclophosphamide has an immunosuppressive effect and is able to suppress the body's excessive immune response. After passing through the liver, cyclophosphamide is converted into several active metabolites that penetrate the cell and tightly connect the two strands of DNA. This prevents tumor cells or other rapidly dividing cells (such as B and T lymphocytes) from dividing. For this reason, cyclophosphamide is included in the treatment regimen for some autoimmune diseases. The subtlety of using steroids, cyclophosphamide and rituximab for autoimmune encephalitis is that these drugs can complicate the diagnosis of those tumors that are associated with the appearance of autoantibodies. In case of lymphoma, the use of these drugs can temporarily improve the condition, but when discontinued, the tumor will again “take up the old ways” and produce autoantibodies. Although lymphoma is not so often complicated by autoimmune encephalitis, this feature must be taken into account during therapy.
Using autoimmune encephalitis as an example, we can consider two of the most important trends in modern medicine. The first trend is the study of the influence of immune processes on the functioning of the human nervous system and attempts to intervene in this process in the treatment of neurological and mental diseases. We are now close to understanding that the brain is not just an electrochemical laboratory under the skull, but also a special immune world with its own rules. It is in our interests to learn these rules and begin to play by them to our advantage. The second trend is the biologization of psychiatry, the search for specific biological changes in the body that lead to mental health problems. Psychiatry is a part of medicine, a large branch where theoretical knowledge and its practical application are combined. Without a biological basis, medicine will cease to be scientific. If you ignore biological laws and knowledge within one of the medical specialties, then the same thing will happen to it. Psychiatry must become biological in order to remain scientific and fulfill its medical goals.
New Horizons
Recently, researchers and doctors have been paying more and more attention to innate immune responses during the development of neurodegenerative diseases (Alzheimer’s disease [24], Parkinson’s disease [25], Huntington’s chorea [26]) and mental disorders [27]. The accumulation of beta-amyloid, which itself is most likely involved in the immune responses of the central nervous system [28], is associated with an imbalance between its accumulation and elimination (removal). The latter process depends in part on how toll-like receptor 2 (TLR2) is expressed [29]. Mouse models have shown that the lower the level of TLR2 expression, the worse the removal of beta-amyloid [30]. Decreased elimination of beta-amyloid leads to its accumulation in nerve cells and subsequent disruption of their function and death. Studying the internal immune processes of the brain can become the basis for the search for new drugs for neurodegenerative diseases. Pharmaceutical companies are now testing monoclonal antibodies to treat Alzheimer's disease, but success remains modest.