Competition “Bio/Mol/Text”-2020/2021
This work was published in the “Free Topic” category of the “Bio/Mol/Text” competition 2020/2021.
The general partner of the competition is the annual biotechnology conference BiotechClub, organized by the international innovative biotechnology company BIOCAD.
The sponsor of the competition is SkyGen: a leading distributor of life science products on the Russian market.
Competition sponsor: the largest supplier of equipment, reagents and consumables for biological research and production.
"Book" sponsor of the competition - "Alpina Non-Fiction"
Disclaimer
Biomolecule doesn't usually publish papers on the same topics, but this year we accepted two papers based on the same 2021 Nature publication. The article you are reading is a review article, but here is its news counterpart [16].
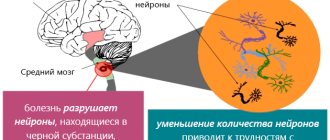
Parkinson's disease is the second most common neurodegenerative disorder. It is characterized by the death of neurons in the substantia nigra of the brain. Current therapy aims to limit disease control. There is still no effective cure. A recent study may radically change this deplorable situation. Hao Qian from the University of California, San Diego (USA), together with colleagues from Peking University (China), demonstrated the successful conversion of astrocytes into neurons in situ in a mouse model of Parkinson's disease. However, let's start with the general characteristics of this disease.
New in the treatment of Parkinson's disease
European research is being conducted in the areas of soft pulsed electrical stimulation of neurons and gene therapy. But as long as the causes of Parkinson's disease remain unclear, no cure will be found to completely cure it. Currently available treatment helps improve quality of life and delay the progression of the disease, so that patients who have recently been diagnosed with this disease have every chance of maintaining the ability to self-care for many years.
Parkinson's disease is one of the most serious illnesses for older people. In order to alleviate its manifestations and improve the general well-being of the patient, professional care and a solid material and technical base are required. Modern geriatric centers can provide all this.
Parkinson's disease: history and molecular pathogenesis
In 1817, the English physician James Parkinson [1] published “An Essay on the Shaking Palsy,” in which he described the neurological disease, and also carried out an analysis and identified general patterns. The name reflects the unusual combination of the main symptoms - tremors, muscle stiffness and inability to maintain balance [2].
For a long time, medicine did not understand the causes of this disorder. A breakthrough in the study of Parkinson's disease occurred already in the 20th century.
By the way, one of the scientists who helped unravel the mysteries of the pathogenesis of Parkinson’s disease was the Russian neuropathologist Konstantin Nikolaevich Tretyakov, who in 1916–1923 held the position of head of the Charcot Brain Laboratory of the Department of Nervous Diseases of the University of Paris (France). He proposed the nigral theory of the pathogenesis of Parkinson's disease, according to which the manifestations of the disease were associated with the loss of the substantia nigra of the brain.
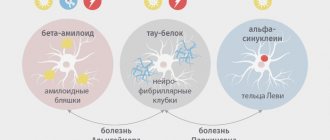
Today we know that Parkinson's disease develops due to the degeneration of dopaminergic neurons in the substantia nigra, which leads to a decrease in the production of dopamine, a neurotransmitter responsible for feelings of satisfaction and attachment. Clinical manifestations are associated with a neurotoxic effect: as the disease progresses, the protein alpha-synuclein accumulates in cells [3]. Alpha-synuclein is normally involved in the transport and release of vesicles containing neurotransmitters. However, in patients with Parkinson's disease, phosphorylation and abnormal folding of this protein are observed with the formation of aggregates - Lewy bodies (Fig. 1). In 2021, a group of scientists from the Scripps Research Institute (USA) discovered that the phosphorylated form of alpha-synuclein is localized in mitochondria, causing their fragmentation [4].
Figure 1. Lewy body in a neuron of the substantia nigra of the brain
[15]
Internal medications for Parkinson's disease
In Parkinson's disease, nerve cells die en masse. The process accelerates if a person experiences stress and negative emotions, since in such cases the blood supply to the brain is disrupted, which is why he does not receive adequate nutrition and a sufficient amount of oxygen. Decoctions and infusions will have a calming effect, improve the nutrition and respiration of neurons, thereby slowing down pathological changes. You should not expect quick action, since medicinal plants are characterized by a cumulative effect. The first results will become visible in a month, but a lasting effect will be obtained only after 6-12 months. At home, it is recommended to take treatment courses 3-4 times a year, alternating monthly doses of the drug with breaks.
Herbal teas
Teas are prepared from linden, chamomile, thyme, oregano, yarrow, sage, St. John's wort, alternating them. To prepare a healing drink, dry herb leaves are brewed with boiling water and left for 10 minutes. Every morning it is recommended to drink a cup of hot aromatic tea.
Medicinal infusions
Grind 3 tablespoons of herbs (St. John's wort, nettle or sage), pour them into a thermos and steam with boiling water. Leave for 30-60 minutes. Take 60-80 milliliters 3 times a day before meals.
Infusions relieve tremors and stiffness, help overcome depression and fear, improve memory and mental activity.
Oatmeal for Parkinson's
A glass of oats is washed well and filled with a liter of water at room temperature. Leave for 9 hours, and then boil for half an hour over low heat. Wrap the dishes in a towel to keep warm and leave for another 12 hours. Drink 100 milliliters three times a day before meals.
Oatmeal decoction cleanses blood vessels and reduces trembling of the limbs, strengthens the musculoskeletal system.
Possibilities of today's therapy
Now there is only symptomatic treatment aimed at alleviating the existing manifestations of the disease. One of the most effective and widely used drugs is levodopa, or 3-hydroxy-L-tyrosine [5]. Levodopa is a biological precursor of dopamine. In addition to levodopa, a number of other drugs are sometimes used. Dopamine receptor agonists have a chemical structure similar to dopamine, and due to this they can stimulate its receptors [6]. Monoamine oxidase B inhibitors can block the release of dopamine and prolong its action. Catechol-O-methyltransferase inhibitors inhibit the breakdown of ingested levodopa and prolong its duration of action in the body.
Also used for the treatment of Parkinson's disease are so-called neurotrophic factors - regulatory proteins that support the viability and functioning of neurons. Although many studies have been conducted on the use of neurotrophic factors in clinical practice, they have several limitations. First, they need precise delivery to their destination. For this purpose, special catheters are used. Secondly, it is advisable to use neurotrophic factors only if the patient has not yet damaged the axons of dopaminergic neurons. If the transport system in the substantia nigra is disrupted or destroyed, then such treatment will not give any positive effects [7].
Some of the above methods of combating the disease are widely used, but none of this will help return a person’s lost neurons. Therefore, there have been attempts to develop therapies aimed at restoring the population of dopaminergic neurons. In 2021, a group of scientists from the Japanese University of Kyoto [8] transplanted induced pluripotent stem cells (iPSCs) [9], capable of turning into any cell in the body, into the patient’s brain. Before iPSCs were used to treat Parkinson's disease in humans, studies of the effectiveness and safety of this therapy were conducted in primates [10]. IPSCs were removed from seven people for their subsequent transformation into neural cells. For transformation, double suppression of the SMAD signaling pathway, which is involved in the processes of cell growth, differentiation and death, was used. The Sonic hedgehog (Shh) signaling pathway is responsible for the specialization of dopaminergic neurons in the substantia nigra. Suppression of SMAD and activation of Shh led to the differentiation of iPSCs into dopaminergic neuron precursor cells, which began to synthesize neural markers [11]. However, stem cell transplantation is not used to treat Parkinson's disease in humans due to its complexity and insufficient evidence of effectiveness.
Parkinson's disease (PD) is a chronic progressive brain disease, predominantly characterized by the degeneration of dopaminergic neurons of the substantia nigra with the formation of special intracellular inclusions in them (Lewy bodies) and manifested by a combination of hypokinesia with rigidity, resting tremor and postural instability [1, 5, 6, 20 ]. In addition to classical motor disorders, PD is characterized by a wide range of non-motor manifestations, including mental, autonomic, sensory symptoms, sleep and wakefulness disorders [2, 24].
One of the common non-motor manifestations of PD is pain. The frequency of pain in patients with PD, according to various authors [12, 16, 19, 21, 25], ranges from 40 to 70% and exceeds that in the general population (10-40%). In approximately 10% of patients, pain is the initial symptom of PD, preceding motor disorders, but it most often occurs on the side of future motor symptoms [24, 27]. At the onset of PD, pain and muscle soreness in the glenohumeral region, especially the biceps brachii muscle, are especially common [18]. Pain is often ignored by doctors caring for patients with PD, but it is often the main symptom that reduces the patient’s quality of life [21].
Despite the high frequency of pain, its causes, relationship with the disease itself, and approaches to its correction remain poorly developed.
The purpose of this study is to determine the frequency of chronic pain syndrome and develop approaches to its diagnosis and systematization.
Material and methods
The study included 130 patients with PD, 57 men and 73 women, with an average age of 63.5±9.1 years and an average disease duration of 3.8±2.3 years. The diagnosis of PD was made in accordance with the clinical diagnostic criteria of the UK PD Society Brain Bank. The stage of the disease on the Hoehn-Yahr scale ranged from 2 to 4 and averaged 2.4±0.7; UPDRS score: 46.7±15.6 points.
The patient selection criterion was the absence of dementia according to ICD-10 criteria.
Pain syndrome was detected in 68 (52%) of 130 patients, 31 men and 37 women, who made up the main group
.
Their average age was 63.1±9.1 years, disease duration was 3.6±2.4 years, and the average Hoehn-Yahr score was 2.5±0.6. The comparison group
included 31 patients with PD without pain - 13 men and 18 women, whose average age was 63.7 ± 9.1 years, disease duration - 3.3 ± 2.8 years, Hoehn-Yahr scale score - 2.3±0.6. There were no significant differences between these groups in gender, age, duration and stage of the disease.
In the main group, 57 (83.8%) patients took levodopa drugs, 49 (72.1%) patients took dopamine receptor agonists, 14 (20.5%) patients took amantadine drugs. In the comparison group, these drugs were taken by 22 (70.9%), 23 (74.1%) and 8 (25.8%) patients, respectively.
To the control group
To assess the threshold of pain sensitivity and the state of vibration sensitivity, 30 practically healthy patients of the same gender and age (14 men and 16 women; average age 66.3 ± 8.8 years) were included as the patients of the main group.
Quantitative assessment of movement disorders was carried out using the Unified Parkinson's Disease Rating Scale (UPDRS), version 3 [14]. Pain assessment using a specially designed daily pain assessment diary (DAD-BP). This diary made it possible to assess the duration and intensity of pain during the day, the relationship with taking levodopa drugs, and the regional distribution of pain. The DOB-BP included a 4-point pain rating: 0—no pain, 1 point—mild pain, 2 points—moderate pain, 3 points—severe pain. The localization of pain was determined by the patient in the following regions of the body: head, neck, torso, lumbar region, upper limbs and lower limbs. The intensity of pain was also determined using a visual analogue scale (VAS), where the subject was asked to rate the pain experienced at the time of examination in points from 0 to 10, where 0 points is no pain, and 10 points is unbearable pain.
The pain threshold was studied using pressalgometry. A cuff was placed on the patient's shoulder, on the inner surface of which a needle applicator was fixed, and when air was pumped into it, the patient experienced a painful sensation. The pain sensitivity threshold was assessed by the pressure in the cuff (in mm Hg) at the moment of pain perception.
For the general assessment of cognitive functions, the Mini Mental State Examination (MMSE) scale was used [15]. To assess individual cognitive functions, we used the “coding” test from the Wechsler Adult Intelligence Scale (WAIS), adapted at the St. Petersburg Psychoneurological Institute. V.M. Bekhterev [4], clock drawing test [26], visual memory test from the SKT scale [22], free and directed association test [2]. The study of health-related quality of life was carried out using the EQ-5D scales [10].
In case of local pain or limited mobility in the spine or joints, radiographic or magnetic resonance imaging (MRI) examination of the relevant structures was done.
Statistical processing was carried out using the Statistica 6.0 software package using the Student's t test, goodness-of-fit test &khgr;2, U test, nonparametric sign test, rank correlation analysis (according to Spearman).
Results and discussion
Of the 68 patients with chronic pain syndrome, in 25 (36.7%) it arose almost simultaneously with the onset of motor disorders or shortly before them, in 43 (63.3%) it developed later, as the disease further progressed. Within a year from the onset of the disease, pain occurred in 25 (36.7%) patients, and a year later - in more than 43 (63.3%) patients.
Pain syndrome was more often localized in the proximal parts of the upper extremities - in 31 (43.9%) patients, than in the distal parts - in 9 (16.3%). This distinguishes it from parkinsonian symptoms, which in PD often initially involve the distal upper extremities. In the upper extremities, pain was most often localized in the shoulder area - in 31 (43.9%) patients, less often in the hand - in 9 (16.3%). Pain in the hip area was detected in 17 (25%) patients, in the lower leg area - in 12 (17.6%), and in the foot - in 10 (14.7%). Pain in the lumbar region was noted by 23 (32.8%) patients. Pain was more often detected or predominated on the side of severe motor manifestations - in 43 (63%) patients, but in 9 (13%) it was more pronounced on the opposite side.
There were no significant differences in the forms of the disease between patients with and without pain: the trembling form was detected in 11.7 and 6.5% of patients, respectively, akinetic-rigid - in 36.8 and 32.2%, mixed - in 51.5 and 61.3%. However, patients with pain syndrome had a higher score on part III of the UPDRS, mainly due to hypokinesia and postural instability, but there was no difference in the severity of tremor and the degree of rigidity (Table 1)
.
At the same time, a correlation was found between the intensity of the pain syndrome, assessed according to the DOB-BP, and the assessment according to the III part of the UPDRS ( r
= 0.43,
p
< 0.01), as well as the severity of hypokinesia (
r
= 0.3,
p
< 0, 05).
Despite the fact that in the whole group there was no dependence of the pain syndrome on the level of rigidity, in 26 (38%) patients pain could be associated with changes in muscle tone (rigidity and dystonia) due to the coincidence of their localization. This type of pain was more often detected in the extremities (25 patients), sometimes involving them in the hemitype, less often in the neck area.
The intensity of the pain syndrome, assessed by DOB-BP and VAS, also depended on the age of the patients at the onset of the disease. Thus, in patients with an earlier onset it was higher ( r
=-0.38,
r
=-0.41,
p
<0.01), while the intensity of pain did not depend on the duration of the disease.
In patients with pain, the mean dose of levodopa was higher (297.0 ± 207.8 mg) than in patients without pain (202.5 ± 177.3 mg), which likely reflects the greater severity of parkinsonian symptoms.
Motor fluctuations were detected in 11 (16.2%) patients of the main group and 5 (16.1%) patients of the comparison group. Although there were no differences in the frequency of pain between patients with and without pain, a significant correlation was found between the VAS pain score and the UPDRS motor fluctuation score (part IV) ( r
=0.32,
p
<0.001). According to the DOB-BP data, in 14 (21%) patients (including 3 people who did not have obvious motor fluctuations), pain depended on the phase of action of the levodopa drug: it arose or intensified during the period of weakening of the effect of the previous dose and decreased at the height of the effect next dose of levodopa.
Patients with pain syndrome had a lower pain sensitivity threshold than patients without pain syndrome (Table 2)
.
In addition, the pain threshold on the side with more severe parkinsonian symptoms was lower compared to the pain threshold on the other side.
The discovered fact is significant evidence that pain is directly related to the main mechanisms of PD development. In 35 (51%) patients, clinical examination, radiography or MRI revealed musculoskeletal pathology associated with degenerative-dystrophic lesions of the spine (25 patients), shoulder joint (5), hip joint (4), knee joint (3). However, only in 19 (27.9%) patients it could be recognized as the main cause of chronic pain syndrome. In such cases, examination revealed local muscle soreness and limited mobility not associated with rigidity or dystonia.
In 13 (19%) patients with PD, an unusual type of pain was observed, which could not be associated either with musculoskeletal pathology, or with local or regional changes in muscle tone, or with daily fluctuations in the effect of levodopa drugs, although it was “congruent” with the predominant distribution of the main symptoms parkinsonism was usually noted in this case. This type of pain was characterized by “fuzzy” localization, a deep aching nature of the pain, and a higher intensity (the average VAS score was 7.4 points, while the score for pain associated with changes in muscle tone was 5.6 ± 1. 4 points), combination with such subjective sensory complaints as numbness or paresthesia, the presence of a peculiar localized component of akathisia (although a full-blown akathisia syndrome was not noted in any case). Pain syndrome with similar characteristics in patients with PD has been described by some authors as “primary” or “central” pain [8]. In no case did the localization of pain correspond to the distribution of symptoms characteristic of diseases of the peripheral nervous system (for example, polyneuropathy) [8]. None of the patients met the criteria for restless legs syndrome [7].
Although there were no differences in overall cognitive performance assessed by the MMSE between the groups of PD patients with and without pain, patients with pain had greater impairment on the clock-drawing, coding, language, and language tests. visual memory (Table 3)
.
The results obtained indicate that patients with pain syndrome have more pronounced cognitive impairments, mainly of a neurodynamic and regulatory nature. In general, the identified impairments correspond to the level of moderate cognitive impairment. There was no correlation between pain intensity and performance on neuropsychological tests.
Symptoms of depression were observed in 49 (72%) patients with pain syndrome and in 11 (35%) patients without pain syndrome. The average Beck score in patients with pain syndrome was higher than in patients without pain (16.8±8.5 and 13.6±7.2 points, respectively, p
<0.01).
Moreover, the severity of pain, assessed by the DOB-BP and VAS, correlated with the severity of depressive symptoms, determined by the Beck scale (respectively, r
= 0.56,
r
= 0.51;
p
< 0.001). However, the pain syndrome in patients with severe depression had no clinical features.
The level of quality of life, determined by the EQ-5D scale, was lower in patients with pain syndrome than in patients without pain syndrome ( p
<0.05).
At the same time, patients with pain in PD have reduced daily activities, increased levels of anxiety and depression, as well as pain and discomfort. The assessment of pain syndrome according to the DOB-BP negatively correlated with the level of quality of life assessed according to the EQ-5D ( r
= -0.57;
p
<0.01).
To correct the main symptoms of parkinsonism and motor fluctuations in 49 (72.1%) patients with pain syndrome, the dose and frequency of levodopa administration were increased: the average daily dose of levodopa was increased by 118.8 ± 22.1 mg and amounted to 415.8 ± 225 .1 mg. In 17 (34%) patients, after dose adjustment of levodopa, the DOB-BP score decreased from 4.9±3.4 to 4.1±2.7 points ( p
<0.05). A positive reaction to increasing the dose of levodopa was noted in pain syndromes associated with fluctuations, local or regional increases in muscle tone, as well as in conditionally identified “central” pain.
Thus, the temporal relationship of the pain syndrome with the course of the disease, its main motor and non-motor manifestations, changes in the threshold of pain sensitivity, as well as a positive reaction to antiparkinsonian therapy allow us to conclude that, at least in the majority of patients with PD, chronic pain syndrome is pathogenetically associated with the disease.
Based on the study, 4 main signs (criteria) can be identified that allow us to establish a connection between pain syndrome and PD: 1) the development of pain simultaneously with the onset of the disease and/or against the background of increasing symptoms of parkinsonism; 2) correspondence of the localization (“congruence”) of pain to the distribution of basic motor manifestations (the predominance of pain on the side of more pronounced symptoms of parkinsonism); 3) reduction of pain when prescribing or correcting antiparkinsonian therapy or its connection with motor fluctuations, phases of action of levodopa drugs or dyskinesias; 4) the absence of other reasons that can explain the pain syndrome.
Pain associated with PD can be identified with a clear positive response to antiparkinsonian therapy, including when associated with fluctuations in the effect of levodopa. There were grounds (presence of the 3rd criterion) or in the presence of the 1st, 2nd and 4th criteria. Based on these criteria, it was possible to associate pain syndrome with PD in 24 (35.3%) patients.
Analysis of clinical phenomenology made it possible to conditionally identify 3 common types of pain syndrome associated with PD: 1) pain with motor fluctuations; 2) pain due to local (regional) changes in muscle tone (rigidity or dystonia); 3) “central” pain. In addition, in 25 (36.8%) patients, only one of the criteria for the connection of pain syndrome with PD was identified (excluding the 3rd criterion). In these cases, pain was stated to be “conditionally associated with PD.” At the same time, its cause could be arthrosis of large joints and degenerative changes in the spine, but nevertheless it undoubtedly worsened due to the development of PD and its manifestations such as rigidity and postural deformities. Pain not associated with PD was noted in 19 (27.9%) patients. It was characterized by a lack of temporal connection with the course of the disease, a discrepancy between the localization and distribution of parkinsonian symptoms, and a lack of response to antiparkinsonian drugs. Its main cause was vertebral and/or articular pathology.
The causes of pain in PD remain poorly understood. Pain syndrome in PD is explained by dysfunction of various levels of the nervous system from peripheral nerve fibers to the cerebral cortex [8]. The experiment showed that the basal ganglia play an important role in processing sensory information and their dysfunction, caused by insufficiency of the dopaminergic system, can lead to a deficiency of descending inhibitory pathways, which modulate the passage of impulses along the somatosensory pain pathways [8, 11, 25]. This is confirmed by the connection between pain and a decrease in the threshold of pain sensitivity demonstrated by a number of researchers and confirmed by our data, as well as the tendency towards its normalization under the influence of dopaminergic therapy noted in a number of works [9, 13, 17]. Perhaps an important role in the development of pain syndrome is played by impaired sensorimotor integration [2]. In recent years, involvement of peripheral sensory fibers in the degenerative process has been revealed in patients with PD, but the clinical significance of this remains unclear [23]. In none of the cases we observed were we able to identify clinical features typical of peripheral neuropathic pain (distribution according to the polyneuropathic type, the presence of allodynia or hyperalgesia, etc.).
Since there is no evidence of direct damage to the somatosensory system in PD, there is no reason to designate pain associated with PD as “neuropathic” in accordance with the modern definition of the latter [8]. However, given the possible role of subcortical structures in the development of pain, pain associated with PD can be defined as “neurogenic.”
Approaches to the treatment of pain in patients with PD depend on its suspected cause. The presence of pain should always be considered as a reason to assess the adequacy of antiparkinsonian therapy. This is especially important if there are criteria for linking pain syndrome with PD. If pain occurs or intensifies during periods of weakening of the effect of levodopa drugs, appropriate correction of antiparkinsonian therapy is necessary, aimed at shortening the “off” period (increasing the dose or frequency of taking levodopa, adding a dopamine receptor agonist, MAO type B inhibitor or COMT inhibitor). If depressive symptoms are detected, their correction is necessary (dopamine receptor agonists, antidepressants). For so-called “central” pain, increasing dopaminergic therapy does not always lead to a reduction in pain. In such cases, the most promising is the prescription of antidepressants (having a noradrenergic component of action), but their effectiveness should be tested in special studies. Finally, if pain is associated with concomitant pathology of the musculoskeletal system, its treatment is carried out according to general rules and should include a complex of physical methods of influence, non-steroidal anti-inflammatory drugs, central muscle relaxants, etc.
In conclusion, it should be noted that pain in patients with PD is a frequent and heterogeneous phenomenon, the clarification of the nature of which allows us to outline the path to its differentiated therapy.
Astrocytes - a possible cure?
Recently, several scientific papers have been published on the transformation of various cells into dopaminergic neurons. Basically, the researchers chose astrocytes - cells that support the vital activity of neurons. They got their name from their characteristic star-shaped shape (Fig. 2). The responsibilities of these cells include providing the metabolic needs of neurons, participating in the timely release of neurotransmitters from nerve endings, storing nutrients and regulating neuronal activity.
Figure 2. Auxiliary cells of the nervous system - astrocytes. Normally, they ensure the vital activity of neurons, but by influencing the molecular processes occurring in astrocytes, they can be “transformed” into neurons of any type.
Human Astrocytes
But most of all scientists are interested in their reparative function. In 2014, a group of scientists from Lund University and Karolinska Institutet discovered that when nerve tissue is damaged after a stroke, astrocytes are able to replace dead neurons [12]. At the same time, the Notch1 signaling pathway, which is of key importance in the processes of cell proliferation and differentiation, “turns off” in them. In a healthy brain, this pathway is active and blocks the conversion of astrocytes into neurons. However, after a stroke, this mechanism is suppressed, and astrocytes can begin to transform into neurons.
The idea for the study, conducted by a team from the University of California, is not new. Back in 2021, a group of Swedish scientists turned human astrocytes into neurons in vitro using viral vectors. After this, mouse astrocytes were also transformed, but in vivo. For reprogramming, they used three transcription factors (NEUROD1, ASCL1 and LMX1A) and miR218 microRNA [13]. Astrocyte transformation in vitro has been improved using molecules capable of inducing chromatin rearrangement and activating several signaling pathways.
However, a 2021 study proposes a much simpler method for reprogramming astrocytes: simply blocking the production of a single protein.
Indications for surgery
In cases of severe motor fluctuations that are not amenable to pharmacotherapy, and especially drug-induced dyskinesias, neurosurgical treatment methods are recommended. They are performed by doctors in partner clinics located in Moscow. Neurosurgeons treat patients with parkinsonism using the stereotactic cryogenic method. They perform cryodestruction of the ventrolateral nucleus of the thalamus and subthalamic region using a special device. After surgery, patients experience complete or almost complete disappearance of tremor and muscle rigidity, and functional capabilities and ability to work are restored.
Indications for surgery are:
- insufficient effectiveness of antiparkinsonian drugs;
- drug dyskinesias;
- poor tolerance to medications;
- pronounced, incurable tremor with medication.
At the Yusupov Hospital, patients are provided with European level services. Doctors use modern treatment regimens for the disease with original, most effective drugs. If you need help from a neurologist specializing in the treatment of Parkinson's disease, call the phone number.
Mechanism of astrocyte transformation
PTB1 as a target for astrocyte reprogramming [14]. This protein is synthesized in astrocytes and inhibits differentiation into neurons. Decreased production of PTB1 causes the production of its neural variant nPTB1 .
First, the scientists transformed astrocytes in vitro. Astrocytes were isolated from mouse midbrain and cortex and from human cortex. The researchers used a hairpin RNA to the Ptbp1 gene, which encodes the PTB1 protein. Hairpin RNAs work on the principle of RNA interference: they interact with the messenger RNA of a specific gene and cause its degradation, resulting in the cell's inability to produce protein. The level of PTB1 in astrocytes dropped, which led to the transformation of astrocytes into neurons.
The researchers then moved on to in vivo experiments: They depleted the PTB1 protein in the mouse brain. Symptoms of Parkinson's disease in mice were induced using a toxic dopamine analogue, 6-OHDA, which causes the death of dopaminergic neurons. Qian and colleagues used transgenic mice producing cre recombinase. This allowed the viral vector to be targeted directly to astrocytes. To make sure that the virus reached its target, a red fluorescent protein gene was inserted into it. The viral vector contained a small hairpin RNA (shPTB) that blocked the Ptbp1 gene. This strategy also led to the conversion of astrocytes into neuronal cells and the restoration of motor activity (Fig. 3).
Figure 3. Left: Mouse astrocytes. Right: neurons obtained through astrocyte reprogramming.
One-Time Treatment Generates New Neurons, Eliminates Parkinson's Disease in Mice
Classification of pain
There are several classifications of Parkinson's pain, the most popular is the division into acute and chronic forms, while chronic can be visceral and central. Also, some scientists adhere to the division by localization.
According to the degree of connection with Parkinson's disease, they distinguish related (with rigidity) and indirectly related (appearing as a result of bedsores, falls and fractures, etc.). So, for example, if Parkinson’s arm hurts, then this may be an associated symptom caused by nerve or muscle lesions or indirectly associated, appearing against the background of a bruise after a fall as a result of loss of coordination of movements.
According to Levin and Makhnev, vertebrogenic, radicular and reflex syndromes are distinguished. And according to Drake - myogenic, dystonic, radicular-neuritic, articular and rare generalized.
There are also daytime or night pains, the latter are characterized by the inability to sleep or frequent awakenings, associated with akinesia or, conversely, periodic involuntary movements of the limbs.
In 19% of cases, an unusual form of the disease occurs, which can be identified by highly intense aching pain in the internal organs, which is combined with severe muscle tension.
results
Qian and colleagues found that depletion of PTB1 in astrocytes stimulates their transformation into neurons. The outcome of this transformation depends on the region of the brain in which the target astrocytes are located. Astrocytes in the midbrain were found to synthesize low levels of the transcription factors Lmx1a and Foxa2. These factors are markers of dopaminergic neuron precursors during midbrain development. However, depletion of PTB1 protein increased the production of these transcription factors in midbrain astrocytes. On the other hand, exposure to cortical astrocytes led to an increase in the synthesis of transcription factors characteristic of cortical neurons.
After 4 weeks of exposure to astrocytes in vitro, 50–80% of the cells became morphologically neuron-like and tested positive for the neuronal markers TUJ1 and MAP2.
In in vivo experiments, 3 weeks after shPTB administration, about 20% of cells that received red fluorescent protein synthesized the neuronal marker NeuN. After 10 weeks, there were already about 80% of cells with NeuN. At the same time, the cells stopped producing GFAP, a protein present in astrocytes.
After 12 weeks, 30–35% of these cells had transformed into dopaminergic neurons. In other words, reprogramming astrocytes helped restore more than 600 dead dopaminergic neurons (Figure 4).
Figure 4. Brain of a mouse with “unilateral” Parkinson's disease. Above - before reprogramming using shPTB. Below - after. Dopaminergic neurons are shown in green.
One-Time Treatment Generates New Neurons, Eliminates Parkinson's Disease in Mice
Moreover, the newly created neurons began to actively produce dopamine. Exposure to 6-OHDA reduced neurotransmitter levels to 25% of normal levels. After therapy, dopamine concentrations rose to 65% of normal levels.
Psychotherapy
There is no doubt that Parkinson's disease significantly changes a person's life. It is very difficult to cope and come to terms with these changes. Often patients need the help of a psychotherapist. Only working with a good specialist will help overcome depression. Maintaining good spirits is one of the primary tasks facing a patient with such a diagnosis. Doctors have long noticed that the success of treatment largely depends on the patient’s mood.
General recommendations
People with Parkinson's disease will have to reconsider their entire lifestyle. For example, you need to pay special attention to your diet. Digestion in this disease suffers significantly, patients often complain of constipation. To avoid this, you need to include in your diet as many fiber-rich foods as possible - vegetables and fruits, brown rice, bran bread, beans. We must not forget about proteins - best in the form of lean meat and fish, as well as eggs. Vitamins are required for normal metabolism, especially vitamins C and E. It is necessary to drink as much water as possible, since dehydration is common in Parkinson's disease.
The regimen for taking prescribed medications is also important. They must be taken exactly on the clock according to the doctor's recommendation.
Will there be a new therapy for Parkinson's disease?
It is too early to talk about the prospects of using astrocyte reprogramming to treat Parkinson's disease in real patients. However, we can already say that this study has helped to understand how best to approach the creation of new directions for the treatment of this disease. Although this promising therapeutic strategy may be applicable to other neurodegenerative diseases in the future, the authors themselves point out that “applying our approach to humans will require overcoming many obstacles, including age-related reprogramming limitations and potential side effects.” In addition, further experiments need to determine whether the reprogrammed cells retain their functional activity over long periods of time.
Medicines
The selection of medications for the treatment of Parkinson's disease depends on the stage of the disease. At the very beginning of the development of the disease, doctors use drugs that stimulate the synthesis of dopamine, a neurotransmitter, the amount of which decreases in parkinsonism. Drugs are also used that stop the breakdown of this neurotransmitter, prevent its reuptake, and stimulate the corresponding receptors in the brain. These drugs are in many ways similar to antidepressants.
In the early stages of the disease, dopamine receptor agonists are used based on the following active ingredients:
- pramipexole;
- ropinirole;
- piribedil;
- rotigotine;
- MAO inhibitors type B (active ingredient rasagiline;
- activator of dopamine release from neuronal depot - amantadine.
At the third of five stages of disease development, a drug with the active substance levodopa is prescribed. If the disease debuted after the age of 70 years, then, as a rule, a levodopa-based drug is prescribed immediately.