Article for the “bio/mol/text” competition: Nowadays it’s difficult to find a person who has never heard of Alzheimer’s, Parkinson’s or Huntington’s diseases. These diseases belong to a group of neurodegenerative diseases that cause the death of neurons and the gradual destruction of the brain. Unfortunately, all of them are incurable. Therefore, scientists are actively working to uncover the mechanisms of development of these diseases and find therapy that will help save patients. In our study, we addressed a still little-studied question: what happens to the synaptic connection of neurons during the neurodegenerative process? The results of this work open a new direction for developing a cure for Huntington's disease and other neurodegenerative diseases.
Competition "bio/mol/text"-2013
This work took first place in the “Own work” category of the “bio/mol/text” competition 2013.
The competition is sponsored by the visionary Thermo Fisher Scientific. The sponsor of the People's Choice Award is Helicon.
As life expectancy increases, more and more people are suffering from Alzheimer's disease and Parkinson's disease. Unfortunately, years of research have not yet led scientists to discover the causes of the development of these diseases and possible therapy. This is mainly due to the fact that almost nothing is known about the factors that cause the disease, and also because very few patients have a genetic predisposition. Most often, these diseases are sporadic, i.e. the reasons for their occurrence have not been established. This leads to endless debate - no one knows how to artificially induce this disease in model animals for experiments and drug discovery. Therefore, more and more scientists are turning their attention to genetic diseases of the nervous system, such as Huntington's disease (HD). This disease, like Alzheimer's disease and Parkinson's disease, belongs to the group of neurodegenerative diseases, with which it shares a number of similar features: death of neurons in the central nervous system, accumulation of amyloid-like protein aggregates, cognitive and motor impairments in patients. At the same time, HD has an important advantage from the point of view of researchers, because it is known which mutation causes this disease. This makes it possible to create precise genetic models and study them in animals. This is important because if we understand the pathogenesis of Huntington's disease, it will be easier for us to understand sporadic neurodegenerative diseases. This is what we tried to do in our study.
Huntington's Chorea Clinic
Treatment of the disease should be carried out only under the guidance and supervision of doctors at a psychiatric clinic. A genetically complex disease can manifest itself differently at any time, so it is important not to miss the moment when symptoms begin to appear. A Huntington's chorea clinic should specialize in neurological disorders in humans.
A slowly progressing disease of the nervous system and cerebral cortex is incurable, but a person with such a diagnosis must be constantly under the supervision of a specialist who can correctly calculate and apply a medication in an emergency. Sick people require careful care, as well as the supervision of doctors such as psychiatrists, neurologists, psychologists, ophthalmologists and orthopedists.
Huntington's disease
Huntington's disease (HD, also “Huntington's disease” in Russian literature) is a hereditary disease of the nervous system that affects approximately 1 in 10 thousand people. The disease was first described by George Huntington in 1872, and has since been named after him, but the clinical symptoms of this disease were known in the 16th century under the name “chorea” (from the Latin choreus - dance). Signs of chorea included involuntary, uncoordinated rapid movements similar to convulsions; This is exactly how modern doctors describe the motor disorders characteristic of HD. The disease can sometimes last up to twenty years, but the outcome is invariably the same: the patient loses the ability to move independently, speak, and then think. Typically, symptoms of Huntington's disease begin between the ages of 30 and 50, although 5–10% of patients experience the onset of symptoms before the age of 20—the so-called juvenile form of the disease [1].
The first symptom of Huntington's disease is involuntary twitching of the limbs, torso and facial muscles. Quite often they are accompanied by sudden mood swings, depression, irritability, slurred speech and clumsy movements. As the disease progresses, these symptoms include difficulty or pain when swallowing, unsteady gait, loss of balance, impaired thinking and memory impairment. Eventually, the patient loses the ability to move without the help of others and usually dies from pneumonia, cardiac arrest or other complications.
An important feature of HD for doctors and researchers is that the disease is hereditary and is caused by a mutation in a single gene. It turned out that the development of HD is caused by an increase in the number of repeats of the CAG triplet encoding glutamine in the first exon of the huntingtin protein gene. Moreover, the greater the number of repeats of this triplet, the earlier the development of the disease begins. Normally, there are from 10 to 35 repeats in the human population. In patients with HD, the number of repeats can be from 36 to 121, in the juvenile form - from 50 and above [2]. Thanks to the identification of the genetic basis of the disease, diagnosing HD is currently not a problem; In addition, prenatal diagnosis of the disease and testing of embryos before implantation during IVF have become possible, which allows even carriers of the mutant gene to have healthy children.
Unfortunately, identifying the exact mutation still does not allow scientists to determine the cause of Huntington's disease and find appropriate treatment. The appearance in a cell of a mutant gene and, accordingly, an altered (mutant) protein can lead to the development of pathology in two ways: loss-of-function or gain-of-functin. In the first case, the mutant protein cannot perform the same function as a normal protein, and this leads to disruption of cellular processes. In the second case, the mutant protein interferes with the normal functioning of the cell, beginning to perform some kind of “extra function.” To understand what happens in HD, scientists are intensively studying both the function of the normal huntingtin protein and the behavior of its mutant form [3].
Unfortunately, attempts to determine the precise cellular function of huntingtin have so far been unsuccessful. Various studies indicate the involvement of this protein in a wide range of biological processes, including the transport of proteins and vesicles (membrane transport vesicles), cytoskeletal organization, clathrin-mediated endocytosis, postsynaptic signaling, transcription regulation and anti-apoptotic processes [4]. If it can be proven that disruption of any of these functions is key to the development of the disease, then drugs to support this function could save patients with Huntington's disease.
If the gain-of-function hypothesis is correct, special attention should be paid to the behavior of the mutant form of huntingtin. It turned out that the mutant protein forms aggregates, which are one of the characteristic features of HD development in both humans and model animals (see sidebar). At first, aggregates were described only in the nucleus, but subsequent work also revealed them in the cytoplasm and processes of neurons [5]. In recent years, many authors are inclined to believe that the formation of aggregates has a rather protective function, and the main pathogenic form of mutant huntingtin is a monomeric soluble protein [6].
Prevention of Huntington's chorea in adults at home
“Currently,” says neurologist Valentina Kuzmina, “rehabilitation using a comprehensive program of therapeutic measures with training of relatives by a team of specialists is applicable: a physical therapy doctor, a physiotherapist, a speech therapist, a clinical psychologist and a neurologist.
Since Huntington's chorea is a hereditary disease, prenatal diagnosis or preimplantation genetic testing of embryos (PGT-M), that is, medical genetic counseling of the family, can be offered as prevention.
Models for studying Huntington's disease
Animal models of HD appeared more than 30 years ago. The first were models based on the introduction of neurotoxic substances into the striatum (for example, quinolinic acid, an NMDA receptor agonist), which caused neuronal death. Currently, most researchers are working on transgenic animal models, which include not only mice and rats, but also invertebrate animals - the fly Drosophila melanogaster and the worm Caenorhabditis elegans.
Mouse models of Huntington's disease differ from each other in the number of CAG repeats and the level of expression of the transgene - the artificially introduced huntingtin gene. Because The development of HD depends on these factors; different strains of mice differ from each other in the rate of development of pathologies. The most widely used models include the R6/2, R6/1, and YAC128 mouse strains, which were also used in our work. In mice of these lines, the symptoms of the disease are most pronounced and appear quite quickly. In addition, these animals develop cognitive and motor impairments with age and develop partial loss of neurons in the striatum and cortex.
Another way to model HD is to use cell culture. In the simplest case, cell cultures with stable transfection of the huntingtin gene are used. For example, these are PC12 cells containing an inducible transgene of the first exon of huntingtin or striatal neurons expressing huntingtin fragments of different lengths. In addition, primary cultures from neurons from transgenic mice or immortalized neurons can be used.
Causes
Genetic aspects . Increase in the number of CAG repeats - triplets of the huntingtin gene (143100, HD gene, IT15, 4p16-3, Â). Paternal transmission of the gene, as well as an increase in the number of repeats, leads to a more severe form of the disease, early onset and rapid progression.
Pathogenesis . Progressive death of nerve cells. A marked decrease in the content of neurotransmitters (g - aminobutyric acid, glutamate decarboxylase, substance P, enkephalins) in the basal ganglia. A marked decrease in the activity of the mitochondrial respiratory chain in the caudate nucleus.
Pathomorphology . Macroscopically: atrophy of the caudate nucleus and dilatation of the ventricles. Microscopically: gliosis and neuronal death, especially in the caudate nucleus and putamen.
Why we decided to study the parameters of synaptic transmission in Huntington's disease
Synaptic transmission is the transmission of signals between neurons using synaptic contact. When one neuron is excited, its synaptic ending releases a mediator into the synaptic cleft - a chemical substance that exerts its excitatory or inhibitory effect on the synaptic ending of the second neuron (Fig. 1). Thus, synapses connect neurons with each other, ensuring the normal functioning of neural networks and the entire nervous system. If any of the brain systems stops functioning, the reason may lie either in a disruption in the functioning of individual neurons, or in a disruption in the communication between them, i.e. disruption of synaptic transmission.
Figure 1. Schematic representation of the synapse structure.
"Wikipedia"
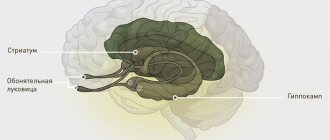
Huntingon's disease affects a specific area of the brain called the striatum. The striatum is part of an important neural pathway, the extrapyramidal system, which is involved in controlling movement and maintaining muscle tone. The death of striatal neurons in Huntington's disease leads to the destruction of the extrapyramidal system, which is associated with loss of control over movements in the sick person. But when the first pathological symptoms appear (tremor, loss of coordination), the human brain is not yet damaged: neurons begin to die only several years after the onset of the disease. Those. the disease begins when something changes in the functioning of the neurons themselves or in synaptic transmission, and these disturbances subsequently lead to the death of neurons and irreversible consequences.
The research results accumulated in recent years make many scientists inclined to believe that it is the disruption of the normal functioning of the neuronal communication system, synapses and synaptic transmission that leads to early disturbances in the functioning of the extrapyramidal system. It turned out that neurons with mutations in the gene encoding the huntingtin protein exhibit a number of pathological changes that disrupt synaptic transmission. In such mutant cells, the formation and renewal of the supply of vesicles (vesicles with a mediator) is disrupted, the intracellular concentration of calcium, which is necessary for the normal release of the mediator into the synaptic cleft, changes, the amount of a number of proteins necessary for the functioning of the synapse is reduced, etc. [7]. All this leads to a reduced release of the transmitter into the synaptic cleft, and if there is not enough transmitter, then the neurons begin to “hear” each other worse, and the commands sent by the cerebral cortex will not be carried out to the fullest extent.
In 2013, the Nobel Prize in Physiology or Medicine was awarded to works that made clear the details of vesicular transport - the process of formation and transportation of membrane vesicles (vesicles) between cells: “Nobel Prize in Physiology or Medicine (2013): vesicular transport” [8]. - Ed.
The study of impaired synaptic transmission in HD was the topic of our study. Could it be that the malfunctioning of striatal neurons in the early stages of HD is caused by the fact that they do not “hear” the commands of cortical neurons? Can weakening of synaptic connections lead to irreversible changes in striatal neurons and lead to their death? What we learned while searching for answers to these questions is described below.
Medical and social examination
Medical and social examination and disability in Huntington's choreaDefinition
Huntington's chorea (CH) is a progressive hereditary degenerative disease, the main clinical signs of which are choreic hyperkinesis, dementia and a progressive course. Prevalence in different countries ranges from 3.2 to 17.4 per 100,000 population. The significance of the disease is also determined by the early social maladjustment of patients. The vast majority of them are recognized as disabled people of group II or I. Etiology and pathogenesis of CG refers to expansion diseases that arise as a result of dynamic mutations. The essence of a mutation is the expansion (repetition) of the same trinucleotide sequences. The appearance of mutations in a gene increases the ability for its further mutation, which determines the phenomenon of anticipation - an earlier onset and a more severe course of the disease in descendants. The hCG gene is mapped to the short arm of chromosome 4. It contains a region of DNA in which the nucleotide sequence is represented by an increased number of triplets, indicating expansion. The pathomorphological substrate of the disease is atrophy of the subcortical extrapyramidal ganglia and cerebral cortex. The weight of the subcortical nuclei decreases by 50%, the weight of the entire brain - by 25%. Small cells of the putamen and caudate nucleus (cholinergic and GABAergic neurons) atrophy predominantly. In the cerebral cortex, atrophy of small cells is noted, Betz cells are not affected. Degeneration of the GABAergic strionigral tract is also detected. It is assumed that a decrease in acetylcholine and GABA neurons leads to an imbalance of neurotransmitters towards the predominance of dopamine mediation. Treatment with drugs that increase the content of GABA in the brain (for example, valproic acid drugs) has no effect, and L-DOPA increases hyperkinesis. At the same time, treatment with dopamine antagonists clearly reduces the severity of hyperkinesis, which is why haloperidol and other antipsychotics are widely used. Clinical features and diagnostic criteria The disease is inherited in an autosomal dominant manner, with high expressivity and penetrance. Due to the late clinical manifestation of hCG, the early death of parents may give the impression of sporadic cases. The average age of onset of the first symptoms of the disease is 38 years, the average duration of the disease is 13-15 years, and life is 52 years. However, in 10% of cases, the disease begins at the age of about 18 years, and sometimes earlier - the so-called juvenile, akinetic-rigid variant. As a rule, the first symptoms of hCG are fussiness, excessive mobility, and absent-mindedness. At the beginning of the disease, hyperkinesis occurs episodically as paroxysms, limited to a certain group of muscles, most often facial muscles. The main type of hyperkinesis is choreic, reminiscent of sudden expressive movements, gesticulation, grimacing, twitching of the nose, snoring, sighs. As the disease progresses, the tonic component, athetoid shade of movements, and then choreoathetosis increase. Speech disorder manifests itself in dysarthria and unnecessary sounds. Muscle tone is low or normal. Sometimes there are autonomic disorders and hypothalamic symptoms (bulimia, polydipsia). Reflexes and sensitivity are not affected. In the akinetic-rigid form, the pathological dominant gene is transmitted from the father. Clinically manifested by akinetic-rigid syndrome, combined with mild limited choreic hyperkinesis and increasing dementia. The progression is faster, the prognosis is worse than with the classic version. The second symptom of hCG is mental disorders. There is no parallelism between the degree of mental defect and the severity of hyperkinesis, although it usually occurs earlier. Mental disorders are manifested by disturbances in the affective sphere, paranoid-hallucinatory psychoses with vivid visual, tactile, auditory hallucinations, and delusional ideas. In the premorbid period, mental abnormalities are observed in 30-60% of patients even before the onset of hyperkinesis (intellectual-mnestic decline, psychopathic behavior, social inferiority). The most characteristic is the psychoorganic syndrome, which in the later stages of the disease reaches the level of dementia. Dementia in CG is characterized by a relatively slow progression, as a result of which patients are hospitalized late, maintaining their ability to work within the framework of simple habitual activities. In the final stage of hCG, a weakening or disappearance of hyperkinesis may be observed with an increase in stiffness and an increase in muscle tone. Diagnostic criteria: - autosomal dominant inheritance; - middle or old age of onset of the disease (with the akinetic-rigid variant, onset is possible in childhood or adolescence); - progressive choreic hyperkinesis; - progressive mental disorders. Additional research data: 1) results of molecular genetic research using direct DNA diagnostics (Ivanova-Smolenskaya I.A., 1995, 1996); 2) data from medical genetic counseling; 3) EEG - low-voltage bioelectrical activity is detected, sometimes at an early stage of the disease; 4) experimental psychological research (state of intellectual-mnestic functions). Subcortical type dementia is typical; 5) CT, MRI - atrophy of the cortex, changes in the configuration of the anterior horns of the lateral ventricles due to atrophy of the caudate nucleus; 6) PET - decreased glucose metabolism in the caudate nucleus (even at an early stage of the disease); 7) consultation with a psychiatrist. Differential diagnosis 1. With chorea of pregnancy (usually a relapse of minor chorea suffered in childhood). 2. With choreic syndrome caused by L-DOPA, sometimes by contraceptives. 3. With hepato-cerebral dystrophy. 4. Akinetic-rigid form - with parkinsonism. 5. With hysterical hyperkinesis. Course and prognosis The clinical and social prognosis in all cases is unfavorable - the disease is steadily progressing. The increase in the severity of CG and the appearance of the first symptoms at a younger age from generation to generation can be used to assess the rehabilitation potential. Patients usually die from intercurrent illnesses. Principles of treatment The most proven direction in the treatment of hCG is the suppression of dopaminergic transmission. In addition to reserpine, which blocks dopamine in presynaptic depots, phenothiazines (aminazine) and butyrophenones (haloperidol, trisedyl), which inhibit postsynaptic dopamine receptors, are used. The use of these drugs, primarily haloperidol, can achieve temporary improvement and delay the progression of the disease in many patients. Due to the need for long-term use and possible complications, doses should be moderate: aminazine no more than 150 mg per day, haloperidol no more than 15 mg per day. For the akinetic-rigid form of hCG, the drugs L-DOPA and parlodel (bromocriptine) are used with some success. Nootropics and Cerebrolysin are used as additional agents in the treatment of hCG. There may be indications for hospitalization of patients with chronic hepatitis in a psychiatric hospital (for acute psychotic disorders) or in a specialized psycho-institution due to severe social maladjustment. Medical and social examination Criteria for VUT The basis for VT is an inpatient examination to clarify the diagnosis and develop a treatment regimen (about 1 month). With decompensation and clear progression of the disease, working patients are temporarily unable to work (usually with subsequent referral to BMSE). Characteristics of disability 1. Motor disorders. It is advisable to distinguish 4 degrees of severity of hyperkinesis in chronic hepatitis, which, along with a mental defect, determines the state of vital activity and labor capabilities of patients: 1) Mild hyperkinesis. Violent movements occur only when performing complex subtle movements; sometimes objectification is possible during stress tests. The gait is not impaired, the patient copes with his previous work and takes care of himself completely. Mild local hyperkinesis can also be detected during fatigue due to heavy physical exertion or significant emotional stress. 2) Moderate hyperkinesis. Small amplitude, usually local, violent movements (in the face, in the hand, while walking) are clearly visible. Observed while moving, during many normal activities. In case of excitement or physical fatigue, they can move to other muscle groups, but never take on the character of a motor storm. Corrective techniques are effective. Movement is free, but tests (walking along a given straight line, with eyes closed) reveal pronounced limitations. The same can be noted when writing and sketching. There may be mild changes in speech. Self-care is preserved, although in general life activity is limited (patients experience some difficulties with complex manipulations with locks and household appliances, and do not perform their household duties efficiently and quickly enough). 3) Severe hyperkinesis. Violent movements occur mainly during movement, but, widespread, are periodically observed at rest, at times (especially in the case of excitement) reaching the level of a motor storm. The “dancing gait” is clearly expressed, articulation disorders are significant (speech is difficult to understand), writing is impossible. At home, patients need outside help, as dexterity, the ability to control the body, and the ability to communicate are significantly reduced. Everyday self-service (dressing, personal toilet, food), although not of sufficient quality, is preserved. 4) Sharply expressed hyperkinesis. Constant widespread (generalized) choreic and choreoathetotic violent movements often occur at rest. Walking is impossible, speech is absolutely incomprehensible. In everyday life, the patient needs constant assistance in all activities (including personal hygiene, eating, dressing). 2. Mental disorders can significantly limit life activity, including at an early stage of the disease. The ability to control the situation and behavior decreases. With dementia and psychosis, orientation is impossible, and therefore patients are incapable of self-care and need constant help and supervision. Indications for referral to BMSE 1. Virtually all patients with diagnosed chronic hepatitis (1-4 years from the onset of the disease, depending on the nature of work activity). Contraindications to continuing the same work are very extensive due to hyperkinesis, which is almost always combined with a mental defect. In fact, only people performing simple and auxiliary work can remain in their previous jobs, and even then with a significant reduction in its volume. 2. Patients with severe and pronounced hyperkinesis and mental disorders. Minimum required examination when referring to BMSE 1. Data from medical genetic analysis. 2. Experimental psychological research. 3. Examination by a psychiatrist. 4. X-ray of the skull. 5. EEG. 6. General blood and urine tests. Disability criteria The main criteria for MSE in chronic hepatitis are the prevalence and severity of hyperkinesis, the severity of mental disorders, the rate of progression, and social factors. Group III: moderate limitation of life activity due to mild (moderate) hyperkinesis and mental disorders (according to the criterion of limited ability to work of the first degree). Group II: significant limitation of life activity with progression of the disease, severe widespread hyperkinesis, distinct mental disorders (according to the criteria of limited ability to self-care, movement, control of one’s behavior of the second degree). Group I: pronounced hyperkinesis, disease outcome in extrapyramidal rigidity, dementia, leading to pronounced impairment of life activity (according to the criteria of limited ability to move, communicate, control one’s behavior, and self-care of the third degree). Subject to observation of disabled people of groups I and II for 5 years, it is determined indefinitely. The cause of disability is usually a general illness. Prevention of disability 1. Primary prevention: prenatal diagnosis (possible in the first trimester of pregnancy). 2. Secondary prevention: a) timely diagnosis, clinical observation, constant pathogenetic therapy; b) in the case of preclinical diagnostics (testing for the hCG gene) - professional reorientation, acquisition of a profession, taking into account possible contraindications. 3. Tertiary prevention: a) prevention of compensation failures in working patients; b) timely determination of disability depending on the degree of disability; c) implementation of other social assistance measures. Rehabilitation An individual rehabilitation program is drawn up for patients with relatively slow progression of the disease. 1. Medical rehabilitation: regular pathogenetic drug therapy. 2. Vocational rehabilitation: a) employment of group III disabled people in simple, better than usual jobs (distributing tools, packing and sorting at an individual pace, cleaning the premises, working in a small wardrobe, etc.); b) some disabled people of group II can be employed at home, patients with a predominance of mental disorders are employed in occupational therapy workshops.
3. Social rehabilitation: a) supply of means of transportation (bicycle stroller, wheelchair), technical means of caring for the patient; b) psychological assistance to the patient’s family, training in caring for a seriously ill patient with chronic hepatitis.
Source
Research Findings: Changes in Synaptic Transmission in Huntington's Disease
Synaptic transmission can be studied in various ways. For example, this can be done by penetrating the neural circuit using electrophysiological methods. A neuron expresses its activity using an electrical current that can be measured. If an experimenter takes a chain of two neurons and, after activating one neuron, records the electrical activity of the second, he can find out how well the signal travels. Another way to study the functioning of synaptic transmission is to study the morphology of the neuron. The fact is that many neurons (including neurons of the cortex and striatum) have special membrane outgrowths - spines, which they need specifically for the formation of synapses (Fig. 2). The more actively a neuron “communicates” with its neighbors, the more spines there are on its surface. Taking these two approaches, we decided to investigate how synaptic transmission works in HD.
Figure 2. Dendritic spines on the surface of a striatal neuron. Spines are small projections on the surface of neuronal processes; in the enlarged image they are marked with arrows.
photo of the author of the article
Figure 3. Neuronal culture from cortical and striatal neurons. Using specific antibodies, cortical neurons are colored red, and striatal neurons are colored yellow-green.
photo of the author of the article
As a model for studying Huntington's disease, a cell culture from neurons of the cortex and striatum was used. To prepare the culture, immature neurons from the studied areas of the mouse brain are planted in Petri dishes, where they form full-fledged neuronal processes and neural chains (Fig. 3). Wild-type mice (without mutations) and YAC128 mice, which carry a mutation in the huntingtin protein gene and are a recognized model of HD, were used. On days 14–15 after neurons are planted in a Petri dish, they reach a mature state corresponding to the state of neurons in the adult brain, and on days 19–20 neurons are considered “old”: they exhibit a number of cellular processes characteristic of the brain of elderly people. In addition, with age, mutant huntingtin protein and its aggregates accumulate in neurons of YAC128 mice, so studying neuronal culture at these two stages provides insight into what is happening in the brain of a patient with HD at the early and late stages of the disease.
Figure 4. Different types of spines on the surface of a dendrite - micrograph and schematic representation.
To begin with, we examined the morphological differences between the two lineages, i.e. compared their appearance. Normal striatal neurons are characterized by the presence of a large number of spines, which is why they are called medium spiny neurons (MSNs). It is the spines that form the majority of synaptic contacts between neurons of the striatum and cortex, and the presence of a certain number of them is important for normal synaptic transmission. The “quality” of spines is also important: in modern neurobiology they are divided into three groups according to size and shape (Fig. 4): mushroom-shaped, thin and stump-shaped. At the same time, different types of spines perform different functions: it is believed that only mushroom-shaped spines form active synapses, while thin and stump spines do not form contacts with other neurons. Thus, for the normal functioning of the neural chain and the effective transmission of information along it, the presence of a certain number of mushroom-shaped spines is necessary.
In order to find out how many mushroom-shaped spines there should be on the SCN normally, a culture of neurons from the brain of wild-type mice was used as a control at all stages of the morphological analysis. It turned out that the number of spines on the SSN of the striatum on day 14 of culture (young neurons) is the same in YAC128 and wild-type cultures, but on day 20 (in “old” neurons) significant changes are observed (Fig. 5). In an “aged” YAC128 culture, the total number of spines decreases, and the relative number of mushroom-shaped spines that form active synapses decreases by half [10]. It turns out that morphological changes in neurons, indicating a violation of synaptic transmission, develop only in “old age” (at a late stage of the disease). This means that in the early stages the root of the problem must lie in another area.
Figure 5. Morphological analysis of striatal neurons. a — neuron spines on days 14 and 20 of culture. Micrographs show areas of neuron dendrites. The relative number of spines of different types is marked on the pie chart: green - mushroom-shaped spines, red - thin spines, black - thin spines. On the 20th day of cultivation, the proportion of mushroom-shaped spines on the surface of YAC128 neurons decreases and the proportion of stump spines increases. b — Dendritic spine density (average number of spines per 10-μm-long dendrite) on wild-type and YAC28 neurons.
Therefore, we compared the electrophysiological characteristics of the two lines. This is possible thanks to the electrical activity of neurons, which can be recorded using a special method called patch clamp. To do this, a thin glass pipette with an electrode inside is applied to the surface of the neuron. When a neuron is activated, the voltage on its cell membrane changes, and this change is recorded by an electrode. If you take two neurons connected by a synaptic contact and, exciting one, record the response electrical activation on the second, you can measure the efficiency of signal transmission through the synapse (Fig. 6). If response activation does not always occur, then synaptic transmission is likely weakened. This method can detect synapse dysfunction before changes in neuron morphology.
Figure 6. Experimental design using combined optogenetics and electrophysiological recording. The cortical neuron is activated when illuminated with blue light, and the response activity is recorded on the striatal neuron in contact with it using a glass pipette with an electrode.
To activate a neuron, you can use several methods. A chemical can be added to the surrounding fluid to open ion channels, causing the neuron to become electrically excited. You can stimulate a neuron with an electrical current. But for our experiments we chose a more subtle tool for influencing neurons, namely optogenetics. This approach is based on the introduction of special light-sensitive proteins - opsins - into neurons, as a result of which the neurons themselves become sensitive to light (Fig. 7). As a result, by illuminating neurons with light of a specific wavelength, their activity can be changed. Illumination with blue light excites the neuron, and with yellow light it causes inhibition (i.e., it suppresses the activity of the neuron).
Optogenetic technologies these days even promise to restore vision to people with degenerative retinal lesions, imparting light sensitivity not to destroyed photoreceptors, but to ganglion cells: “Optogenetics + holography = insight?” [eleven]. - Ed.
Figure 7. Opsins are light-sensitive proteins that enable the movement of ions across the cell membrane and changes in neuronal activity. Channelrhodopsin (ChR2) causes membrane depolarization and neuron activation, halorhodopsin (NpHR) causes membrane hyperpolarization and neuron inhibition.
Genetic testing for Huntington's disease in the HTT gene
An increase in the number of CAG triplet repeats (expansion) in exon 1 of the HTT gene is the cause of the development of Huntington's disease, an autosomal dominant neurological disease.
Synonyms Russian
Huntington's chorea, triplet repeat expansion, HTT gene, genetic testing.
English synonyms
Huntington disease, expansion of CAG (cytosine-adenine-guanine) triplet repeats, HTT gene.
Gene name
HTT gene.
Localization of the gene on the chromosome
Locus 4p16.
Research method
Fragment analysis of exon 1 of the HTT gene.
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
- Do not smoke for 30 minutes before the test.
General information about the study
Huntington's disease (HD) is a hereditary progressive neurodegenerative disease caused by the expansion of CAG triplet repeats in exon 1 of the HTT gene, located on the short arm of chromosome 4 and encoding the huntingtin protein (HTT). A small number of CAG (cytosine-adenine-guanine) nucleotides in the HTT gene are found normally, but with an increase in the number of repeats (expansion) more than 35, the likelihood of developing the disease and transmitting the gene to descendants increases significantly. The size of the expansion correlates with the severity of symptoms and the time of first manifestations of the disease.
The incidence of the disease among populations with European roots is 10:100,000. The disease is inherited in an autosomal dominant manner, that is, there is a 50% risk of developing it in offspring.
Huntington's disease manifests itself predominantly with neurological symptoms. Clinical manifestations and signs:
- Motor disorders - hyperkinesis: chorea, dystonia, athetosis, tremor, myoclonus; oculomotor disorders; bradykinesia and rigidity; dysphagia; impaired balance, coordination and gait.
- Cognitive impairment - decreased executive daily functioning; dementia.
- Neuropsychiatric disorders - depression; irritability, aggressiveness; psychoses (delusions, hallucinations, paranoia, overvalued ideas); apathy; perseveration and obsessive-compulsive disorder.
- Instrumental examination - MRI of the brain: atrophy of the head of the caudate nucleus, manifested by an increase in the frontal horns of the lateral ventricles of the brain. Reduction in the volume of the cerebral cortex. With MR spectroscopy: increased lactate in the occipital cortex; decrease in NAA/creatine index. PET/CT study: hypometabolism of the basal ganglia and frontal cortex.
What is the research used for?
In accordance with international clinical guidelines, genetic testing for Huntington's disease is carried out if the patient has clinical symptoms characteristic of this disease, as well as the patient's relatives and children.
When is the study scheduled?
- If Huntington's disease is suspected;
- in the differential diagnosis of hyperkinesis;
- in the differential diagnosis of chorea and dystonia;
- for cognitive and neuropsychiatric disorders;
- when characteristic changes are detected during an MRI study of the brain;
- with early detection of the disease in relatives;
- when planning a family.
What do the results mean?
Genetic testing is the main method of confirming the diagnosis and is based on counting the number of triple CAG repeats using the fragment analysis method in the HTT gene. The diagnostic significance of the detected number of CAG repeats in the HTT gene is presented in the table:
| Number of CAG repeats in the HTT | Diagnostic test | Predictive test | Developmental Risk for Children |
| 6-26 – normal allele | Huntington's disease ruled out | Huntington's disease will not develop | Low risk of developing Huntington's disease |
| 27-35 – moderate increase | Huntington's disease is very unlikely | It is unlikely that you will develop Huntington's disease | Risk of developing HD ( |
| 36-39 – moderate expansion | The diagnosis of Huntington's disease was confirmed. Possible mild manifestations | Huntington's disease with reduced penetrance may develop | Increased risk of developing Huntington's disease |
| 40 or more – pronounced expansion | Huntington's disease diagnosis confirmed | Huntington's disease will develop | Increased risk of developing Huntington's disease |
What can influence the result?
Although a genetic test is an accurate method of laboratory diagnosis, the time of clinical manifestations of the disease (disease penetrance) depends on the external environment and individual genetic factors. To assess the nature of inheritance in children and relatives, the risk of disease progression and prescribe treatment, it is recommended to consult a specialist.
Important Notes
- To obtain a conclusion based on the results of the examination, it is necessary to consult a clinical geneticist.
Who orders the study?
Neurologist, psychiatrist, geneticist.
Also recommended
[42-054] Genetic testing for Huntington's disease type 2 in the JPH3 gene
[42-055] Genetic testing for Huntington's disease type 4 in the TBP gene
[42-056] Genetic testing for dentatorubro-pallidoluis atrophy in the ATN1 gene
[42-057] Comprehensive examination for huntington-like diseases (HPZ2, HPZ4, DRPLA)
[06-080] Ceruloplasmin
[06-083] Copper in the blood
[06-136] Copper in urine
[42-048] Screening for common genetic causes of cerebellar ataxia (SCA 1,2,3,6,7, AF)
[42-043] Determination of triplet expansion in spinocerebellar ataxia type 1 (in the ATXN1 gene)
[42-044] Determination of triplet expansion in spinocerebellar ataxia type 2 (in the ATXN2 gene)
[42-045] Determination of triplet expansion in spinocerebellar ataxia type 3 (in the ATXN3 gene)
[42-046] Determination of triplet expansion in spinocerebellar ataxia type 6 (in the CACNA1A gene)
[42-047] Determination of triplet expansion in spinocerebellar ataxia type 7 (in the ATXN7 gene)
[13-060] Diagnosis of systemic lupus erythematosus
Literature
- Martha Nance et al., A Physician's Guide to the Management of Huntington's disease (Third Edition), HDSA 2011.
- Robert B. Daroff, Bradley's Neurology in Clinical Practice, 2-Volume Set, 7th Edition, Elsevier, 2021.
- Losekoot M, van Belzen MJ, Seneca S, Bauer P, Stenhouse SaR, Barton DE. EMQN/CMGS best practice guidelines for the molecular genetic testing of Huntington disease. Eur J Hum Ge net. 2013; 21(5): 480-486.
- Bean L, Bayrak-Toydemir P. American College of Medical Genetics and Genomics Standards and Guidelines for Clinical Genetics Laboratories, 2014 edition: technical standards and guidelines for Huntington disease. Genet Med. 2014; 16(12): e2.
Optogenetics
Optogenetics is a method that combines the approaches of genetics and optics for fine control of the electrical activity of electrically excitable cells (neurons and muscle fibers) [9]. To do this, genes of special light-sensitive proteins—microbial opsins, which are ion channels or pumps—are introduced into the cells under study (Fig. 7). The first work showing the possibility of controlling the electrical activity of neurons using opsin was published in 2005. Over the next few years, a number of experimental works appeared that made it possible to refine this technique and prove its applicability under various experimental conditions.
In recent years, many different opsins have been discovered, of which halorhodopsins and channelrhodopsins have found the most application in optogenetics. When the opsin gene is delivered using genetic engineering methods into a neuron, light-sensitive channels appear on the plasma membrane, and the cell itself becomes light-sensitive. When exposed to blue light, the channelrhodopsin pore opens (maximum absorption - 470 nm), which causes the movement of positively charged ions into the cell, ensuring depolarization of the neuron membrane and the generation of action potentials. When exposed to yellow light, halorhodopsin is activated (maximum absorption - 580 nm), the neuron membrane is hyperpolarized, causing inhibition of the neuron. The high temporal resolution of optogenetics allows for very fine regulation of synaptic events and is therefore an important tool for studying interneuronal connections.
The combined use of optogenetics and classical electrophysiology techniques allows us to benefit from the positive qualities of each of these approaches. The precision of electrophysiological recording is combined with the ability to use light stimuli of varying durations and intensities to help scientists study the workings of neural connections in detail.
The electrical activity of neurons is expressed in sharp voltage surges on the cell membrane and the resulting appearance of an electric current. These sudden jumps are called spikes or action potentials and last a few milliseconds (Figure 8). We found that the longer a neuron is illuminated with blue light, the more spikes it creates during this time. If the illuminated neuron is connected by a synaptic contact with another neuron, then response activity can be recorded on the second neuron, also in the form of individual spikes.
Figure 8. An example of a recording obtained from electrophysiological recording of neuron activity. Spikes (action potentials) are reflected in the recording as vertical lines showing sharp jumps in membrane current.
In our experiments, we used a pair of young (14 days) cortical and striatal neurons connected by synaptic contact. The cortical neuron was activated with blue light, and the response activity was recorded on the striatal neuron. It turned out that for a response to occur on a striatal neuron, a certain duration of illumination (activation threshold) is required. If the duration of illumination was below the threshold value, then a spike on a striatal neuron did not occur in response to every flash of light. And most importantly, the activation threshold for neurons from the brains of healthy mice was different from YAC128 mice (with a mutation in the huntingtin gene). This difference was most clearly visible at 50% activation of the striatal neuron, i.e. the duration of illumination at which a spike occurs in response to every second flash of light. The threshold for 50% activation of a striatal neuron in response to irradiation of a cortical neuron for the YAC 128 cell culture was approximately two times (more precisely 2.3 ± 0.8) higher compared to the positive control [10].
It turns out that a mutation in the huntingtin protein leads to the fact that synaptic transmission deteriorates even in young neurons, and the disturbances occur at the functional level (without morphological changes). Could it be that it is these functional disorders that subsequently lead to the appearance of morphological changes, such as the disappearance of spines in old striatal neurons?
To answer this question, we again turned to optogenetics for help, but now with its help we suppressed the activity of neurons with light (Fig. 9a). If our assumption is correct, then the temporary absence of the activating influence of the cortex should lead to the disappearance of spines on striatal neurons in the YAC128 culture. Indeed, after the experiment, the number of spines in the positive control remained unchanged, but in the YAC128 culture it decreased significantly (Fig. 9b, c). It turns out that neurons modeling HD are especially sensitive to weakening the activating influence of cortical neurons , therefore, long-term weakening of the synaptic connection between these neurons leads to a decrease in the number of spines on the 20th day of cultivation [10].
Figure 9. Influencing neurons using optogenetics. a — suppression of cortical neuron activity using optogenetics. When a neuron is illuminated with yellow light (orange stripe), it becomes inactive—there are almost no spikes in the electrophysiological recording during this period of time. b — influence of long-term optogenetic inhibition on the morphology of neurons. Micrographs show a decrease in the number of spines on the dendrite of the YAC128 neuron. c — change in the density of dendritic spines after optogenetic inhibition. In the wild-type culture, the number of spines does not change, but in the YAC128 culture, spine density is significantly reduced.
In summary, we found that in our HD model there is a disruption of synaptic transmission that develops in two stages. At an early stage (young neurons, on day 14 of neuron culture), a functional weakening of the synaptic connection occurs, which at a later stage (old neurons, on day 20 of culture) leads to morphological disturbances of synaptic contacts.
Conclusion: synaptic transmission disorders and neurodegenerative diseases
The study confirmed our initial guess: disruption of the striatum in HD is associated primarily with disruption of synaptic transmission. The striatum neurons stop “hearing” the commands of the cortical neurons, and, as a result, the person begins to lose control over his movements. But at an early stage of the disease, these disorders are functional in nature and are probably reversible. If we find out the reasons leading to disruption of synapses, then it is possible to develop a drug that will help stop pathological changes, provide striatal neurons with the necessary level of activation and, thus, prevent the development of irreversible morphological disorders.
The results of this work also lead to another important conclusion: when studying neurodegenerative diseases, scientists should pay more attention to synaptic transmission and the interaction of neurons. We know quite a lot about how the accumulation of amyloid aggregates in Alzheimer's disease affects cell activity, and which neurons die during the development of Parkinson's disease, but this has still not led to the emergence of effective therapy. A possible reason for these failures is that we do not take into account the consequences of disruption of synaptic connections - those that we discovered in the study of Huntington's disease. We hope that our findings will point researchers in the direction of developing effective treatments for Huntington's disease, and Thirteen will finally be healthy!
The study was carried out in the laboratory of molecular neurodegeneration of St. Petersburg State Polytechnic University (head of the laboratory - Prof. Ilya Borisovich Bezprozvanny, University of Texas) under the guidance of Ph.D. Dmitry Nikolaevich Artamonov.