Multiple sclerosis: safe switching from fingolimod to alemtuzumab
Switching from Gilenya (fingolimod) to Lemtrada (alemtuzumab) during the treatment of relapsing multiple sclerosis - after a short washout period (when no therapy is used to remove the previous drug from the body) - does not lead to has been found to increase the risk of disease reactivation. Moreover, alemtuzumab, promoted by Sanofi, provides significant reduction in inflammation, reflected in a reduction in the number of relapses and new areas of brain damage, when compared with fingolimod, which is promoted by Novartis.
It was previously noted that the transition from Gilenya to Lemtrada in some cases causes a significant and unexpected activation of multiple sclerosis due to insufficient response to alemtuzumab. The supposed reason lies in the relatively short half-life of the latter, when it simply does not have time to deplete the pool of circulating autoreactive lymphocytes that are still sequestered in the lymphoid tissue. The subsequent release of lymphocytes that have “hidden” from the beneficial biological effects of alemtuzumab provokes reactivation of the disease. This phenomenon requires further study in order to deepen the understanding of the methodology of an individualized approach to the development of treatment regimens for multiple sclerosis.
Fingolimod is a modulator of sphingosine-1-phosphate receptors (S1PR), and is nonspecific: it binds to their subtypes 1, 3, 4 and 5 (S1PR1, S1PR3, S1PR4 and S1PR5). Fingolimod, by binding to and stimulating S1P receptors on the surface of lymphocytes, works as a functional antagonist. The result is the internalization and degradation of the desired receptors with a corresponding decrease in their activity. Because expression of S1R receptors allows lymphocytes to follow a gradient of lymphocytes to exit lymphoid organs, fingolimod blocks such activity by isolating (sequestering) the lymphocytes within the lymphoid tissue. The result is the inhibition of lymphocyte migration with subsequent infiltration of the central nervous system, which provides an anti-inflammatory effect due to a powerful decrease in the concentration of circulating T helper 17 (Th17) cells and the effector cytokine interleukin 17 (IL-17) produced by them.
Alemtuzumab binds to the CD52 protein found on the surface of B and T lymphocytes, as well as natural killer cells, monocytes and macrophages, resulting in lymphocyte lysis mediated by an antibody-dependent cellular cytotoxic effect and complement fixation.
The study, carried out by specialists from the University of Cagliari (Italy), set out to uncover the problem: whether switching from fingolimod to alemtuzumab is actually associated with an increased risk of reactivation of multiple sclerosis, or whether this reactivation should be expected during a change in treatment regimen made due to the ineffectiveness of fingolimod.
The analysis included data from 77 patients with relapsing multiple sclerosis who switched to Lemtrada from Gilenya after it stopped working. The average age of participants was 36.2 years; women accounted for 79.2%; the disease lasted an average of 12.3 years. The washout period between the two drugs averaged 1.8 months. Over half of the patients (64%) received more than one course of Lemtrada therapy. Before alemtuzumab was prescribed, more than half (55%) of patients had a normal level of lymphocytes in the blood (>1000 cells/ml) - the rest had a lower number than normal.
"Mosmedpreparaty"
The annualized relapse rate (ARR) during Gilenya treatment was 0.60. During the “washing” period it increased to 1.33. After starting Lemtrada, the ARR dropped to 0.20.
After changing the drug, 14% of patients (n=11/77) experienced one or more relapses. The median time to the first relapse in the “washing out” period was 28 days; after the prescription of Lemtrada it increased significantly - to 315 days.
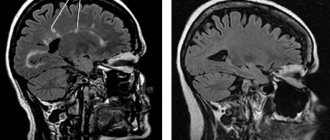
During fingolimod treatment, new brain lesions and new active lesions on T2- and T1-weighted MR images, respectively, were observed in 69.2% and 58.6% of patients. After switching to alemtuzumab, these proportions dropped sharply to 10.4% and 2.2%.
In summary, the researchers noted the absence of high activity of multiple sclerosis in the first year of treatment with Lemtrada prescribed after Gilenya, despite the fact that alemtuzumab provided significant control of the inflammatory process in comparison with fingolimod. This is true even in the case of both a short and long “washing out” period, and a normal number of lymphocytes, which, as is known, are directly involved in the pathophysiology of multiple sclerosis. Thus, the use of Lemtrada soon after stopping Gilenya is not a risk factor for reactivation of the disease. It is stated that it is optimal to switch from one drug to another after a month of pause, so that the number of lymphocytes does not turn out to be too low.
Multiple sclerosis
Alemtuzumab able to repair damaged brain tissue?
October 22, 2008 - An experimental multiple sclerosis drug appears to be far more effective than conventional treatments for multiple sclerosis . But everything good has its price.
Patients with relapsing-remitting multiple sclerosis treated with alemtuzumab experienced fewer exacerbations and fewer signs of progression of multiple sclerosis than those receiving current standard treatment with interferon beta-1a.
Notably, some of the patients treated with the experimental drug had even less disease-related disability three years into the study than at the start of the study. This gives reason to hope that this treatment will make it possible to stop the development of the disease before it has time to lead to severe disabling consequences.
One of the subjects died
However, nearly one in four subjects treated with alemtuzumab developed treatment-related thyroid dysfunction.
Even more alarming was the fact that 3% of those treated with it developed a life-threatening autoimmune disease, which in one case led to death.
One of the study leaders, Dr. Alasdair Coles, said that phase III clinical trials of the drug are planned for the near future, the purpose of which is to determine whether the potential benefits of alemtuzumab in the treatment of patients with relapsing-remitting multiple sclerosis outweigh the risks of its side effects.
According to the Multiple Sclerosis Society of America, relapsing-remitting multiple sclerosis accounts for about 85% of all cases of newly diagnosed multiple sclerosis.
A report on the study results was published in The New England Journal of Medicine on October 23.
“The phase II trial results are exciting, but the drug is not yet ready for widespread clinical use,” says Dr. Coles. “We need to know more about its effectiveness and the side effects that occur with long-term use. This is work for several more years.”
Treat once a year
Developed decades ago by scientists at the University of Cambridge, alemtuzumab was the first monoclonal antibody drug for use in humans. It is officially approved for use in the treatment of chronic lymphocytic leukemia (CLL).
Lemtuzumab works by detecting and destroying a certain type of immune cells that normally serve to protect the body from infections. It is believed that in multiple sclerosis and other autoimmune diseases, the functioning of these cells is impaired, leading to the destruction of healthy tissue.
Initially, Cambridge researchers tested the drug on patients with advanced stages of multiple sclerosis, but the success of these trials was very modest.
The Phase II trial, the results of which have only recently become known, involved only patients with early stages of relapsing-remitting multiple sclerosis who had not previously received other drugs used in the treatment of multiple sclerosis.
Between December 2002 and July 2004, 334 patients from Europe and the United States were recruited to participate in the study.
About 1/3 of the patients received standard therapy with interferon beta-1a, which was administered by injection three times a week. The remainder were treated with alemtuzumab , given as a cycle of intravenous infusions given once a year.
The first cycle included four-hour infusions of the drug daily for five days. After twelve months, most patients received a second cycle of treatment, consisting of three infusions over three days.
Efficiency was unprecedented
Three years after the start of the study, treatment with the experimental drug was clearly associated with a sharp decrease in the number of clinically significant relapses of the disease, as well as a decrease in the activity of the inflammatory process (which was confirmed by brain MRI data) in comparison with interferon therapy.
But the most impressive result of the study, Coles said, was that the experimental treatment reversed damage to brain tissue caused by multiple sclerosis.
“This result can be considered unprecedented. This is a big event,” he says. “Another important part of this approach will be treatment at the earliest possible stage and with the most effective means known today.”
The study was financially supported by Genzyme and Bayer Schering Pharma AG, the owners of commercial rights to alemtuzumab .
At a press conference on Wednesday, Genzyme's medical director, Dr. Susan Moran, spoke about a patient death during the trial.
The patient died from an autoimmune blood disorder known as idiopathic thrombocytopenic purpura (ITP). Dr. Moran believes that the death could have been avoided if ITP had been included in the list of possible side effects of alemtuzumab .
“Unfortunately, the patient had symptoms of ITP but did not seek medical attention until diagnosis because the condition was not listed as a possible side effect,” she said.
Once the risk of this complication became apparent, all patients in the study were carefully screened for ITP. Five more similar cases were identified, which were managed through adequate treatment.
Careful monitoring of patients
Dr. Moran said that all patients who will take part in the phase III trial, as well as those who will be treated with alemtuzumab if it is approved as a treatment for multiple sclerosis , will be carefully monitored for this complication.
Researchers are also working on ways to identify patients at risk for ITP before referral for alemtuzumab treatment. In addition, rules will be developed to select those patients who will benefit most from early aggressive treatment.
In an editorial accompanying the study report, neurologist Stephen L. Houser, who has spent many years studying multiple sclerosis, wrote that it is not yet certain that alemtuzumab will become a first-line drug for the treatment of early stages of multiple sclerosis.
Dr. Hauser is Chief of the Department of Neurology at the University of California, San Francisco Medical Center.
“The cumulative toxicities associated with alemtuzumab have significantly dampened enthusiasm for its widespread use in patients with multiple sclerosis until more complete data are available regarding its long-term safety and durability,” he writes.
Dr. John Richert of the Multiple Sclerosis Society of America (MSA) says it is becoming increasingly clear that aggressive treatment of early-stage multiple sclerosis is a better approach than a wait-and-see approach that allows the disease to progress.
However, he agrees that the potential value of alemtuzumab in treating multiple sclerosis remains to be seen.
Dr. Richert is NARS Vice President for Research and Clinical Programs.
“Alemtuzumab may well be the breakthrough treatment for multiple sclerosis that we are trying to find, but this will only be seen in phase III clinical trials,” he says.
Cancer drug proves to be most effective treatment for multiple sclerosis
2 minutes
1089
A large-scale phase three clinical trial of alemtuzumab, which lasted a total of four years, confirmed its greater effectiveness in improving the condition of patients with relapsing-remitting multiple sclerosis (MS) compared to interferon beta-1a (Rebif), the standard drug used for this disease. Reports from two parallel rounds of testing of alemtuzumab, conducted by researchers at the University of Cambridge (UK), were published November 1 in The Lancet.
Alemtuzumab is a licensed drug that has been used for decades to treat chronic lymphocytic leukemia and T-cell lymphoma. It contains monoclonal humanized antibodies that bind to the CD52 glycoprotein, which is expressed on the surface of normal and malignant B and T lymphocytes, natural killer cells, monocytes and macrophages.
However, over time, it was noticed that alemtuzumab also has a positive effect on the condition of people with MS, which was confirmed during the first clinical trials, also conducted by specialists from Cambridge, which ended in 2008. Scientists believe this phenomenon is based on the fact that alemtuzumab selectively destroys mature lymphocytes involved in the immune response in MS, without affecting their precursors in the bone marrow. After this, the population of immune cells is restored, but no longer contains lymphocytes that attack the myelin sheaths, which resembles a kind of “reboot” of the immune system.
In the latest phase of clinical trials designed to definitively confirm the effectiveness of alemtuzumab in MS, in one case the effect of the drug was compared with Rebif in patients with an early stage of the disease who had not previously received any treatment, and in the second - in those who had not responded to treatment.
In both cases, alemtuzumab was twice as effective in preventing disease relapse as Rebif. In addition, the general condition of patients with advanced MS improved after two years of alemtuzumab compared to the beginning of treatment. According to study leader Dr. Alasdair Coles, none of the new MS drugs on the market in recent years have performed as well as alemtuzumab compared to Rebif.
At the same time, in an interview with the BBC, Coles emphasized that alemtuzumab is simply the most effective drug for patients with relapsing-remitting MS that currently exists, but is by no means a panacea. In addition, clinical trials have revealed quite serious side effects of alemtuzumab. We are talking about various infectious complications, allergic reactions and autoimmune diseases, mainly associated with thyroid dysfunction, as well as immune thrombocytopenia.
According to Coles, alemtuzumab can mainly be used as an alternative drug for those patients who have not responded to standard treatment, and in rarer cases, as a first and main treatment. In addition, Coles emphasized, alemtuzumab is not effective in progressive MS.
The manufacturer of alemtuzumab, Genzyme (a division of the French pharmaceutical giant Sanofi), withdrew the drug from the US and EU markets in August in order to obtain a license for its use as a treatment for MS from the relevant regulators. As The Lancet editorial on the completion of clinical trials for alemtuzumab noted, once licensed, the price of the drug could rise and become too high for most patients and health systems.
Recently, three new drugs have been proposed for MS. In 2010, the drug fingolimod (trade name Gilenya) manufactured by Novartis was released onto the US market. On September 12, 2012, US regulatory authorities approved the drug teriflunomide, marketed by Sanofi under the trade name Aubagio. The European regulator is expected to make a decision on teriflunomide in the first quarter of 2013. On September 21, 2012, two international teams of specialists reported the successful results of the third and final phase of clinical trials of the drug BG-12 (dimethyl fumarate), commonly used in the treatment of psoriasis, as a treatment for MS.
{#vrez.57990}