Midline anterior to motor cortex
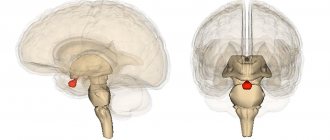
Some motor areas in the human cerebral cortex. The supplementary motor area is shown in pink. Image credit: Pascari
In the supplementary motor zone
(
SMA
) is a part of the primate cerebral cortex that contributes to motor control. It is located on the midline of the surface of the hemisphere just anterior (in front of) the leg representation of the primary motor cortex. In monkeys, the SMA contains a rough map of the body. In humans, the body map is not visible. Neurons in the SMA project directly to the spinal cord and may play a role in direct control of movement. Possible functions attributed to the SMA include postural stabilization of the body, coordination of both sides of the body, such as during bimanual action, control of movements that are internally generated rather than triggered by sensory events, and control of movement sequences. . All of these proposed features remain hypotheses. The exact role or roles of SMA are not yet known.
For the discovery of the SMA and its connections to other motor cortex areas, see the main article on the motor cortex.
Subregions
At least six areas are now recognized within the larger region once defined as the SMA. These divisions have been studied most extensively in the monkey brain. The most anterior portion is now commonly referred to as the pre-SMA.[1][2][3] It has sparse or no connections to the spinal cord or primary motor cortex, and has extensive connections to prefrontal regions.[1][4][5][6][7]
The supplementary eye field (SEF) is the relatively anterior portion of the SMA which, when stimulated, produces head and eye movements and possibly limb and trunk movements.[8][9][10][11]
Dahm and Strick [5] hypothesized on the basis of cytoarchitecture and connections to the spinal cord that the portion of the SMA in the cingulate sulcus, in the mid-hemisphere, may be divided into three distinct regions, the cingulate motor areas. The functions of the motor areas of the cingulate cortex have not been systematically studied.
The SMA proper in monkeys is now limited to the area at the vertex of the hemisphere and extends partly to the medial wall, just before the presentation of the primary motor leg. The SMA itself projects directly to the spinal cord and is therefore one of the main output areas of the cortical motor system.[5][12][13][14][15][16]
Recently, Zhang et al.[17] examined the functional subdivisions of medial SFC based on whole-brain connectivity characterized from a large resting-state fMRI data set. In addition to reproducing the boundaries between the SMA and preSMA, the current results support a functional difference between the posterior and anterior pre-SMA. In contrast to the posterior pre-SMA, the anterior pre-SMA is associated with most prefrontal but not somatomotor areas. Overall, the SMA is strongly connected to the thalamus and epithalamus, posterior pre-SMA to the putamen, pallidum, and the STN and anterior pre-SMA to the caudate nucleus, with the caudates showing significant hemispheric asymmetry.
Features of the structure of the cerebral cortex
The anatomical structure of the cerebral cortex determines its characteristics and allows it to perform the functions assigned to it. The cerebral cortex has the following number of distinctive features:
- neurons in its thickness are arranged in layers;
- nerve centers are located in a specific place and are responsible for the activity of a certain part of the body;
- the level of activity of the cortex depends on the influence of its subcortical structures;
- it has connections with all underlying structures of the central nervous system;
- the presence of fields of different cellular structure, which is confirmed by histological examination, while each field is responsible for performing some higher nervous activity;
- the presence of specialized associative areas makes it possible to establish a cause-and-effect relationship between external stimuli and the body’s response to them;
- the ability to replace damaged areas with nearby structures;
- This part of the brain is capable of storing traces of neuronal excitation.
The large hemispheres of the brain consist mainly of long axons, and also contain in their thickness clusters of neurons that form the largest nuclei of the base, which are part of the extrapyramidal system.
As already mentioned, the formation of the cerebral cortex occurs during intrauterine development, and at first the cortex consists of the lower layer of cells, and already at 6 months of the child all structures and fields are formed in it. The final formation of neurons occurs by the age of 7, and the growth of their bodies is completed at 18 years.
An interesting fact is that the thickness of the cortex is not uniform over its entire length and includes a different number of layers: for example, in the area of the central gyrus it reaches its maximum size and has all 6 layers, and sections of the old and ancient cortex have 2 and 3 layers. x layer structure, respectively.
The neurons of this part of the brain are programmed to restore the damaged area through synoptic contacts, so each of the cells actively tries to restore damaged connections, which ensures the plasticity of neural cortical networks. For example, when the cerebellum is removed or dysfunctional, the neurons connecting it with the terminal section begin to grow into the cerebral cortex.
analyzer, closure apparatus of conditioned reflex connections and working device. Weakness of the closure function of the cortex and trace manifestations can be observed in children with severe mental retardation, when the formed conditioned connections between neurons are fragile and unreliable, which entails learning difficulties.
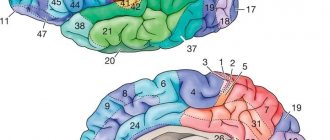
The cerebral cortex includes 11 areas consisting of 53 fields, each of which is assigned its own number in neurophysiology.
Regions and zones of the cortex
The cortex is a relatively young part of the central nervous system, developing from the terminal part of the brain. The evolutionary development of this organ occurred in stages, so it is usually divided into 4 types:
- The archicortex or ancient cortex, due to the atrophy of the sense of smell, has turned into the hippocampal formation and consists of the hippocampus and its associated structures. With its help, behavior, feelings and memory are regulated.
- The paleocortex, or old cortex, makes up the bulk of the olfactory area.
- The neocortex or new cortex has a layer thickness of about 3-4 mm. It is a functional part and performs higher nervous activity: it processes sensory information, gives motor commands, and also forms conscious thinking and human speech.
- The mesocortex is an intermediate version of the first 3 types of cortex.
The cerebral cortex has a complex anatomical structure and includes sensory cells, motor neurons and internerons, which have the ability to stop the signal and be excited depending on the received data. The organization of this part of the brain is built according to the columnar principle, in which the columns are divided into micromodules that have a homogeneous structure.
The basis of the micromodule system is made up of stellate cells and their axons, while all neurons react equally to the incoming afferent impulse and also send an efferent signal synchronously in response.
The formation of conditioned reflexes that ensure the full functioning of the body occurs due to the connection of the brain with neurons located in various parts of the body, and the cortex ensures synchronization of mental activity with the motor skills of organs and the area responsible for analyzing incoming signals.
Signal transmission in the horizontal direction occurs through transverse fibers located in the thickness of the cortex, and transmit the impulse from one column to another. Based on the principle of horizontal orientation, the cerebral cortex can be divided into the following areas:
- associative;
- sensory (sensitive);
- motor.
When studying these zones, various methods of influencing the neurons included in its composition were used: chemical and physical stimulation, partial removal of areas, as well as the development of conditioned reflexes and registration of biocurrents.
The associative zone connects incoming sensory information with previously acquired knowledge. After processing, it generates a signal and transmits it to the motor zone. In this way, it is involved in remembering, thinking, and learning new skills. Association areas of the cerebral cortex are located in proximity to the corresponding sensory area.
The sensitive or sensory area occupies 20% of the cerebral cortex. It also consists of several components:
- somatosensory, located in the parietal zone, is responsible for tactile and autonomic sensitivity;
- visual;
- auditory;
- taste;
- olfactory.
Impulses from the limbs and organs of touch on the left side of the body enter along afferent pathways to the opposite lobe of the cerebral hemispheres for subsequent processing.
Neurons of the motor zone are excited by impulses received from muscle cells and are located in the central gyrus of the frontal lobe. The mechanism of data receipt is similar to the mechanism of the sensory zone, since the motor pathways form an overlap in the medulla oblongata and follow to the opposite motor zone.
The cerebral cortex is formed by several layers of neurons. A characteristic feature of this part of the brain is a large number of wrinkles or convolutions, due to which its area is many times greater than the surface area of the hemispheres.
Cortical architectonic fields determine the functional structure of areas of the cerebral cortex. All of them are different in morphological characteristics and regulate different functions. In this way, 52 different fields are identified, located in certain areas. According to Brodmann, this division looks like this:
- The central sulcus separates the frontal lobe from the parietal region; the precentral gyrus lies in front of it, and the posterior central gyrus lies behind it.
- The lateral groove separates the parietal zone from the occipital zone. If you separate its side edges, you can see a hole inside, in the center of which there is an island.
- The parieto-occipital sulcus separates the parietal lobe from the occipital lobe.
The core of the motor analyzer is located in the precentral gyrus, while the upper parts of the anterior central gyrus belong to the muscles of the lower limb, and the lower parts belong to the muscles of the oral cavity, pharynx and larynx.
The right-sided gyrus forms a connection with the motor system of the left half of the body, the left-sided one - with the right side.
The posterior central gyrus of the 1st lobe of the hemisphere contains the core of the tactile sensation analyzer and is also connected to the opposite part of the body.
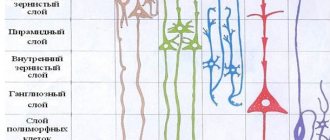
Cell layers
The cerebral cortex carries out its functions through neurons located in its thickness. Moreover, the number of layers of these cells may differ depending on the area, the dimensions of which also vary in size and topography. Experts distinguish the following layers of the cerebral cortex:
- The surface molecular layer is formed mainly from dendrites, with a small inclusion of neurons, the processes of which do not leave the boundaries of the layer.
- The external granular consists of pyramidal and stellate neurons, the processes of which connect it with the next layer.
- The pyramidal layer is formed by pyramidal neurons, the axons of which are directed downward, where they break off or form associative fibers, and their dendrites connect this layer with the previous one.
- The internal granular layer is formed by stellate and small pyramidal neurons, the dendrites of which extend into the pyramidal layer, and its long fibers extend into the upper layers or descend down into the white matter of the brain.
- The ganglion consists of large pyramidal neurocytes, their axons extend beyond the cortex and connect various structures and sections of the central nervous system with each other.
The multiform layer is formed by all types of neurons, and their dendrites are oriented into the molecular layer, and axons penetrate the previous layers or extend beyond the cortex and form associative fibers that form a connection between gray matter cells and the rest of the functional centers of the brain.
Functions
Penfield and Welch[18] in 1951 first described SMA in the monkey and human brain as an image of the body on the medial wall of the hemisphere. Woolsey and colleagues[19] confirmed SMA in the monkey brain in 1952, describing it as a rough somatotopic map with the legs in the back and the face in the front. It was found that the images of different parts of the body overlap greatly. Stimulation of many sites produced bilateral movements and sometimes movements of all four limbs. This overlapping somatotopic map in the SMA has been confirmed by many others.[2][13][20][21][22]
Four main hypotheses for the function of SMA have been proposed: control of postural stability during stance or walking,[18] coordination of temporal sequences of actions,[23][24][25][26][27][28][29][30] bimanual coordination,[31][32] and initiation of internal movement rather than stimulus-driven movement.[3][29][30][33] However, the evidence does not generally support an exclusive role for the SMA in any of these functions. Indeed, the SMA is clearly active during nonsequential, monotonous, and stimulus-driven movements.[34]
For voluntary human movement, the role of the SMA has been elucidated: its activity generates the early component of the Bereitschaftspotential (BP) or readiness potential BP1 or BP at an early stage.[35] The role of SMA was further confirmed by Cunnington et al. 2003,[36] shows that the SMA proper and pre-SMA are active before,before voluntary movement or action, as well as the cingulate,motor area (CMA) and the anterior middle cingulate cortex (aMCC). Recently, by integrating simultaneously acquired EEG and fMRI, it was shown that the SMA and aMCC have strong reciprocal connections that act to maintain each other's activity, and that this interaction is mediated during movement preparation according to the amplitude of the Bereitschafts potential.[37]
SMA in the monkey brain can enhance movement, especially complex movement such as climbing or jumping.[38][39][40] This assumption was based on studies in which stimulation on a behaviorally relevant time scale produced complex whole-body movements resembling climbing or jumping. This hypothesis is consistent with previous hypotheses including the involvement of the SMA in postural stabilization, internally generated movements, bimanual coordination, and movement sequence planning, because all of these functions are significantly involved in complex locomotion. The locomotion hypothesis is an example of interpreting the motor cortex in terms of the underlying behavioral repertoire from which abstract control functions arise, an approach emphasized by Graziano et al.[38]
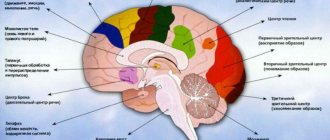
The role of the cerebral cortex
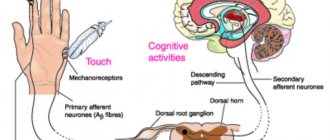
In the posterior central gyrus, behind the central sulcus, there is an area of cutaneous and joint-muscular sensitivity.
Here the signals that arise when touching our body, when it is exposed to cold or heat, or when it is painful are perceived and analyzed. In contrast to this zone, in the anterior central gyrus, in front of the central sulcus, the motor zone is located. It identifies areas that provide movement of the lower extremities, muscles of the trunk, arms, and head. When this area is irritated by electric current, contractions of the corresponding muscle groups occur. Injuries or other damage to the motor cortex lead to paralysis of the body muscles.
The auditory area is located in the temporal lobe. The impulses arising in the receptors of the cochlea of the inner ear are received here and analyzed. Irritation of areas of the auditory zone causes sensations of sounds, and when they are affected by the disease, hearing is lost.
The visual zone is located in the cortex of the occipital lobes of the hemispheres. When it is irritated by electric current during brain surgery, a person experiences sensations of flashes of light and darkness. When it is affected by any disease, vision deteriorates and is lost.
Near the lateral sulcus there is a taste zone where taste sensations are analyzed and formed based on signals arising in the receptors of the tongue. The olfactory zone is located in the so-called olfactory brain, at the base of the hemispheres. When these areas are irritated during surgery or during inflammation, people smell or taste something.
There is no purely speech zone. It is represented in the cortex of the temporal lobe, the inferior frontal gyrus on the left, and parts of the parietal lobe. Their diseases are accompanied by speech disorders.
The cerebral hemispheres occupy about 80% of the volume of the cranium, and consist of white matter, the basis of which consists of long myelinated axons of neurons. The outside of the hemisphere is covered by gray matter or the cerebral cortex, consisting of neurons, unmyelinated fibers and glial cells, which are also contained in the thickness of the sections of this organ.
The surface of the hemispheres is conventionally divided into several zones, the functionality of which is to control the body at the level of reflexes and instincts. It also contains the centers of higher mental activity of a person, ensuring consciousness, assimilation of received information, allowing adaptation in the environment, and through it, at the subconscious level, through the hypothalamus, the autonomic nervous system (ANS) is controlled, which controls the organs of circulation, respiration, digestion, excretion , reproduction, and metabolism.
In order to understand what the cerebral cortex is and how its work is carried out, it is necessary to study the structure at the cellular level.
Recommendations
- ^ a b
He, S.K., Doom, R.P.
and Strick, P.L. (1995). "Topographic organization of corticospinal projections of the frontal lobe: motor areas on the medial surface of the hemisphere." J. Neurosci
.
15
(5):3284–3306. DOI:10.1523 / JNEUROSCI.15-05-03284.1995.CS1 maint: several names: list of authors (link to site) - ^ a b
Luppino, G., Matelli, M., Camarda, R. M., Gallese, V., & Rizzolatti, G. (1991).
"Multiple body movement representations in mesial area 6 and adjacent cingulate cortex: an intracortical microstimulation study in macaque monkeys." J. Comp.
Neurol .
311
(4):463–482. Doi:10.1002/cne.903110403. PMID 1757598.CS1 maint: several names: list of authors (link to site) - ^ a b
Matsuzaka, Y., Aizawa, H., & Tanji, J. (1992).
"The motor area grows into the supplementary motor area (premotor area) in the monkey: neural activity during a learned motor task." J. Neurophysiol
.
68
(3):653–662. doi:10.1152/jan.1992.68.3.653. PMID 1432040.CS1 maint: several names: list of authors (link to site) - Bates, J. F., & Goldman-Rakic, P. S. (1993). "Prefrontal connections of medial motor areas in the rhesus monkey". J. Comp.
Neurol .
336
(2):211–228. Doi:10.1002/cne.903360205. PMID 7503997. - ^ a b c
Doom, R. P., & Strick, P. L. (1991).
"The origin of corticospinal projections from premotor areas of the frontal lobe". J. Neurosci
.
11
(3):667–689. Doi:10.1523/JNEUROSCI.11-03-00667.1991. - Lu, M. T., Preston, J. B., & Strick, P. L. (1994). "Relationships between the prefrontal cortex and premotor areas of the frontal lobe". J. Comp.
Neurol .
341
(3):375–392. Doi:10.1002 / cne.903410308.CS1 maint: several names: list of authors (link to site) - Luppino, G., Matelli, M., Camarda, R., & Rizzolatti, G. (1993). "Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in macaques". J. Comp.
Neurol .
338
(1):114–140. Doi:10.1002/cne.903380109. PMID 7507940.CS1 maint: several names: list of authors (link to site) - Chen L.L. and Walton M.M. (2005). "Head movement induced by electrical stimulation in the rhesus monkey's supplementary eye field." J. Neurophysiol
.
94
(6):4502–4519. doi:10.1152/jan.00510.2005. - Russo, G.S. and Bruce, K.J. (2000). "Supplementary eye field: Representation of saccades and the relationship between neural response fields and evoked eye movements." J. Neurophysiol
.
84
(5):2605–2621. doi:10.1152/jn.2000.84.5.2605. - Schlag, J. & Schlag-Rey, M. (1987). "Evidence for an accessory ocular field". J. Neurophysiol
.
57
: 179–200. doi:10.1152/jn.1987.57.1.179. - Technician, E., & Lee, C. (1993). "The dorsomedial frontal cortex of the rhesus monkey: topographic imaging of saccades evoked by electrical stimulation." Exp.
Brain Res .
96
(3):430–442. Doi:10.1007/bf00234111. - Galea, M. & Darian-Smith, I (1994). “The multiple populations of corticospinal neurons in macaques are determined by their unique cortical origins, spinal endings, and connections.” Cereb.
Bark .
4
(2): 166–194. Doi:10.1093/cercor/4.2.166. - ^ a b
McPherson, J., Marangoz, K., Miles, T.S.
and Wiesendanger, M (1982). "Microstimulation of the supplementary motor area (SMA) in the awake monkey". Exp.
Brain Res .
45
(3): 410–416. Doi:10.1007 / bf01208601.CS1 maint: several names: list of authors (link to site) - Murray, E.A. & Coulter, J. D. (1981). "Organization of corticospinal neurons in the monkey". J. Comp.
Neurol .
195
(2):339–365. Doi:10.1002/cne.901950212. - Nudo, R.J. & Masterton, R. B. (1990). "Descending tracts of the spinal cord, III: sites of origin of the corticospinal tract". J. Comp.
Neurol .
296
(4):559–583. Doi:10.1002/cne.902960405. - Tooshima, K., & Sakai, H. (1982). “The precise cortical extent of origin of the corticospinal tract (CST) and the quantitative contribution to CST in different cytoarchitectonic regions. Study of horseradish peroxidase in monkeys." J. Hirnforsch
.
23
: 257–269. - Zhang, S., Ide, J. S., Li, K. S. (2012). "Resting-state functional connectivity of the medial superior frontal cortex". Cereb.
Bark .
22
(1):99–111. Doi:10.1093/cercor/bhr088. PMC 3236794. PMID 21572088.CS1 maint: several names: list of authors (link to site) - ^ a b
Penfield, W. & Welch, C. (1951).
"The supplementary motor area of the cerebral cortex: a clinical and experimental study". AMA Arch.
Neurol. Psychiatry .
66
(3):289–317. Doi:10.1001/archneurpsyc.1951.02320090038004. PMID 14867993. - Woolsey, C. N., Settlage, P. H., Meyer, D. R., Sencer, W., Hamuy, T. P., & Travis, A. (1952). "Localization patterns in premotor areas and their relation to the concept of the premotor area". Association for Research in Nervous and Mental Diseases
.
New York, NY: Raven Press. 30
: 238–264.CS1 maint: several names: list of authors (link to site) - Gould, H.J. III, Cusick, K.G., Pons, T.P. & Kaas, J. H. (1996). "Relationships of corpus callosum connections with electrical stimulation maps of motor, supplementary motor, and frontal eye fields in owl monkeys." J. Comp.
Neurol .
247
(3):297–325. Doi:10.1002/cne.902470303. PMID 3722441.CS1 maint: several names: list of authors (link to site) - Muakkassa, K.F. And Strick P.L. (1979). "Frontal lobe inputs to primate motor cortex: Evidence from four somatotopically organized 'premotor' areas". Brain Res
.
177
: 176–182. Doi:10.1016/0006-8993(79)90928-4. PMID 115545. - Mitz, A. and Wise, S.P. (1987). "Somatotopic organization of the supplementary motor area: mapping of intracortical microstimulation". J. Neurosci
.
7
(4): 1010–1021. Doi:10.1523/JNEUROSCI.07-04-01010.1987. - Gaymard, B, Pierrot = Deseilligny, C. and Rivaud, S (1990). "Impairment of the sequence of memory-guided saccades after additional lesions of the motor area." Annals of Neurology
.
28
(5):622–626. Doi:10.1002 / ana.410280504.CS1 maint: several names: list of authors (link to site) - Gerloff, K., Corwell, B., Chen, R., Hallett, M. and Cohen, L.G. (1997). “Stimulation of the human supplementary motor area interferes with the organization of future elements in complex motor sequences.” Brain
.
120
(9):1587–1602. Doi:10.1093/brain/120.9.1587.CS1 maint: several names: list of authors (link to site) - Jenkins, I.H., Brooks, D.J., Nixon, P.D., Frackowiak, R.S. and Passingham R.E. (1994). "Motor sequence learning: a positron emission tomography study." J. Neurosci
.
14
(6):3775–3790. DOI:10.1523 / JNEUROSCI.14-06-03775.1994.CS1 maint: several names: list of authors (link to site) - Lee, D. & Quessi, S (2003). "Activity in the supplementary motor area associated with learning and performance during a sequential visuomotor task." J. Neurophysiol
.
89
(2):1039–1056. doi:10.1152/jan.00638.2002. - Musiake, H., Inase, M., & Tanji, J. (1990). "Selective encoding of motor sequence in the supplementary motor area of monkey cerebral cortex". Exp.
Brain Res .
82
: 208–210. Doi:10.1007/bf00230853.CS1 maint: several names: list of authors (link to site) - Shima, K., & Tanji, J. (1998). “Both accessory and supplementary motor areas are critical for the temporal organization of multiple movements.” J. Neurophysiol
.
80
(6):3247–3260. doi:10.1152/jn.1998.80.6.3247. PMID 9862919. - ^ a b
Roland P.E., Larsen B., Lassen N.A.
& Skinhoy, E. (1980). "The supplementary motor area and other cortical areas in the organization of voluntary movements in humans." J. Neurophysiol
.
43
(1):118–136. doi:10.1152/jan.1980.43.1.118. PMID 7351547.CS1 maint: several names: list of authors (link to site) - ^ a b
Roland P.E., Skinhoy E., Lassen N.A.
& Larsen, B. (1980). "Different areas of the human cortex in the organization of voluntary movements in extrapersonal space." J. Neurophysiol
.
43
: 137–150. doi:10.1152/jan.1980.43.1.137. PMID 7351548.CS1 maint: several names: list of authors (link to site) - Brinkman, S (1981). "Lesions in the supplementary motor area prevent the monkey from performing a bimanual coordination task." Neurosci.
Latvian .
27
(3):267–270. Doi:10.1016/0304-3940(81)90441-9. PMID 7329632. - Serrien, D. J., Strens, L. H., Oliveiero, A., Brown, P. (2002). "Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) impairs bimanual motor control in humans." Neurosci.
Latvian .
328
(2):89–92. Doi:10.1016/s0304-3940(02)00499-8. PMID 12133562.CS1 maint: several names: list of authors (link to site) - Halsband, W., Matsuzaka, Y., & Tanji, J. (1994). "Neuronal activity in primate supplementary, pre-supplementary, and premotor cortices during sequential movements with external and internal instructions." Neurosci.
Res .
20
(2): 149–155. Doi:10.1016/0168-0102(94)90032-9. PMID 7808697.CS1 maint: several names: list of authors (link to site) - Pickard, N., & Strick, P. L. (September 2003). "Activation of the supplementary motor area (SMA) during visually guided movements." Cereb.
Bark .
13
(9):977–986. Doi:10.1093/cercor/13.9.977. PMID 12902397. - Dick L., Kornhuber (1978). "supplementary" motor cortex in voluntary human finger movements." Brain Res
.
159
(2):473–476. Doi:10.1016/0006-8993(78)90561-9. PMID 728816. - Cunnington, R., Windischberger, S., Dick, L., & Moser, E. (2003). "Preparation and readiness for voluntary movement: a high-profile event associated with the fMRI study of the Bereitschafts-BOLD response." NeuroImage
.
20
(1):404–412. Doi:10.1016/s1053-8119 (03) 00291-x. PMID 14527600. - Nguyen VT, Breakspear M, Cunnington R (2014). "Reciprocal interactions of the SMA and cingulate cortex support premotor activity for voluntary actions." J Neurosci
.
34
(49):16397–16407. Doi:10.1523/jneurosci.2571-14.2014. PMC 6608485. PMID 25471577. - ^ a b
Graziano, M.S.A.
(2008). Intelligent motion machine
. Oxford, UK: Oxford University Press. - Graziano, M.S.A. & Aflalo, T. (2007). "Mapping of behavioral repertoire to cortex". Neuron
.
56
(2):239–251. doi:10.1016/j.neuron.2007.09.013. PMID 17964243.CS1 maint: several names: list of authors (link to site) - Graziano, M.S.A., Aflalo, T. and Cook, D.F. (2005). "Hand movements evoked by electrical stimulation in the motor cortex of monkeys". J. Neurophysiol
.
94
(6):4209–4223. doi:10.1152/jan.01303.2004. PMID 16120657.CS1 maint: several names: list of authors (link to site)
First and second signaling systems
The role of the cerebral cortex in improving the first signaling system and developing the second is invaluable. These concepts were developed by I.P. Pavlov. The signaling system as a whole is understood as the entire set of processes of the nervous system that carry out perception, processing of information and the response of the body. It connects the body with the outside world.
The first signaling system determines the perception of sensory-specific images through the senses. It is the basis for the formation of conditioned reflexes. This system exists in both animals and humans.
In the higher nervous activity of man, a superstructure has developed in the form of a second signaling system. It is peculiar only to humans and is manifested by verbal communication, speech, and concepts. With the advent of this signaling system, abstract thinking and generalization of countless signals from the first signaling system became possible. According to I.P. Pavlov, words turned into “signals of signals.”
The emergence of the second signaling system became possible thanks to complex labor relationships between people, since this system is a means of communication and collective work. Verbal communication does not develop outside of society. The second signaling system gave rise to abstract (abstract) thinking, writing, reading, counting.
Words are perceived by animals, but completely differently from people. They perceive them as sounds, and not their semantic meaning, like humans. Therefore, animals do not have a second signaling system. Both human signaling systems are interconnected. They organize human behavior in the broad sense of the word. Moreover, the second changed the first signaling system, since the reactions of the first began to largely depend on the social environment. A person has become able to control his unconditioned reflexes, instincts, i.e. first signaling system.
Functions of the cerebral cortex
Familiarity with the most important physiological functions of the cerebral cortex indicates its extraordinary importance in life. The cortex, together with the subcortical formations closest to it, is a department of the central nervous system of animals and humans.
The functions of the cerebral cortex are the implementation of complex reflex reactions that form the basis of higher nervous activity (behavior) of a person. It is no coincidence that it received the greatest development from him. The exclusive properties of the cortex are consciousness (thinking, memory), a second signaling system (speech), and a high organization of work and life in general.
further reading
- Principles of Neuroscience (2000), 4th ed., Kandel et al.
- Debaer, F., Wenderoth, N., Sunaert, S., Van-Heck, P., Swinnen, S. P. (July 2003). "Internal and external movement generation: differential neural pathways involved in bimanual coordination performed in the presence and absence of enhanced visual feedback." NeuroImage
.
19
(3): 764–76. Doi:10.1016/s1053-8119 (03) 00148-4. PMID 12880805.CS1 maint: several names: list of authors (link to site) - Vorobiev; and others. (1998). "Division of human mesial region 6: cytoarchitectonic evidence for three distinct regions". Eur J Neurosci
.
10
(6):2199–203. Doi:10.1046/j.1460-9568.1998.00236.x. PMID 9753106.