pharmachologic effect
Cerebrolysin contains low molecular weight biologically active neuropeptides that penetrate the BBB and directly enter nerve cells.
The drug has an organ-specific multimodal effect on the brain, i.e. provides metabolic regulation, neuroprotection, functional neuromodulation and neurotrophic activity. Metabolic regulation:
The drug Cerebrolysin increases the efficiency of aerobic energy metabolism of the brain, improves intracellular protein synthesis in the developing and aging brain.
Neuroprotection:
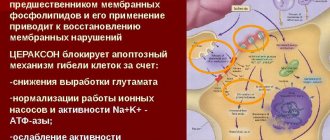
the drug protects neurons from the damaging effects of lactic acidosis, prevents the formation of free radicals, increases survival and prevents the death of neurons under conditions of hypoxia and ischemia, and reduces the damaging neurotoxic effect of excitatory amino acids (glutamate).
Neurotrophic activity:
Cerebrolysin is the only nootropic peptidergic drug with proven neurotrophic activity similar to the action of natural neuronal growth factors (NGF), but manifested under conditions of peripheral administration.
Functional neuromodulation:
The drug has a positive effect in cases of cognitive impairment on memory processes.
Dosage
The drug is used parenterally. The dose and duration of use depend on the nature and severity of the disease, as well as the age of the patient. A single administration of the drug in a dose of up to 50 ml is possible, but a course of treatment is more preferable.
The recommended course of treatment is daily injections for 10-20 days.
| Indication | Dose |
| Acute conditions (ischemic stroke, traumatic brain injury, complications of neurosurgical operations) | from 10 ml to 50 ml |
| In the residual period of cerebral stroke and traumatic injury to the brain and spinal cord | from 5 ml to 50 ml |
| Psychoorganic syndrome and depression | from 5 ml to 30 ml |
| Alzheimer's disease, dementia of vascular and combined Alzheimer's-vascular origin | from 5 ml to 30 ml |
| In neuropediatric practice | 0.1-0.2 ml/kg body weight |
To increase the effectiveness of treatment, repeated courses can be carried out as long as the patient's condition improves as a result of treatment. After the first course, the frequency of injections can be reduced to 2 or 3 times a week.
Cerebrolysin is used parenterally in the form of intramuscular injections (up to 5 ml) and intravenous injections (up to 10 ml). The drug in a dose of 10 ml to 50 ml is recommended to be administered only through slow intravenous infusions after dilution with standard solutions for infusion. The duration of infusions ranges from 15 to 60 minutes.
Side effects
The frequency of adverse reactions was determined in accordance with WHO recommendations: very often: (≥1/10); often: (from ≥1/100 to <1/10); uncommon (from ≥1/1000 to <1/100); rare (from ≥1/10,000 to <1/1000); very rare, including isolated reports (<1/10,000).
From the immune system:
very rarely - increased individual sensitivity, allergic reactions.
Mental disorders:
rarely - the expected activation effect is accompanied by agitation, manifested by aggressive behavior, confusion, and insomnia.
From the nervous system:
rarely - too rapid administration of the drug can lead to dizziness; very rarely - isolated cases of generalized epilepsy and one case of seizures were associated with Cerebrolysin.
From the cardiovascular system:
very rarely - too rapid administration of the drug can lead to increased heart rate and arrhythmia.
From the digestive system:
very rarely - dyspepsia, diarrhea, constipation, nausea, vomiting; rarely - loss of appetite.
For the skin and subcutaneous tissues:
very rarely - skin reactions; rarely - with excessively rapid administration, a feeling of heat, sweating, and itching may occur.
General disorders and disorders at the injection site:
very rarely - redness, itching, burning at the injection site, pain in the neck, head and limbs, fever, mild back pain, shortness of breath, chills, collapsing state.
One study reported an association between rare use (>1/10,000 to <1/1,000) with hyperventilation, hypertension, hypotension, fatigue, tremor, possible depression, apathy and/or somnolence, and flu-like symptoms. (cold, cough, respiratory tract infections).
Since Cerebrolysin is used mainly in elderly patients, the above symptoms of diseases are typical for this age group and often also occur without taking the drug.
It should be noted that some undesirable effects (excitement, arterial hypertension, arterial hypotension, lethargy, tremor, depression, apathy, dizziness, headache, shortness of breath, diarrhea, nausea) were identified during clinical studies and occurred equally in patients treated with Cerebrolysin and in patients in the placebo group.
If any of the side effects indicated in the instructions are aggravated or any other side effects not specified in the instructions are noted, the patient should inform the attending physician.
Notification in case of suspected side effects
It is important to report side effects after drug approval to ensure ongoing monitoring of the drug's risk-benefit profile. Healthcare professionals are asked to report all cases of adverse reactions observed with the drug through national adverse reaction reporting systems and/or to the company's representative office.
"Cortexin"
This drug is not known to many, but doctors often prescribe it to their patients. The drug is most often produced in the form of a liquid for intramuscular administration, so under no circumstances should you inject it yourself without a prescription and the help of a doctor, and besides, it is sold only by prescription.
Indications for use of the drug
- Violation of intracerebral circulation . This may apply not only to the brain, but also to the spinal cord. Symptoms may include: regular headaches or migraines, numbness, nausea, frequent dizziness, and a feeling of stuffiness in the ears.
- Encephalopathy . Only doctors can make this diagnosis. There are a couple of symptoms that manifest the disease, such as fatigue, sleep disturbance, severe memory loss and many others.
- Dystonia . This diagnosis cannot be called a disease, because it is an innate feature of the body that people live with and do not complain about, but there are very pronounced signs that you want to get rid of as quickly as possible. For example, difficulty breathing, dizziness, low blood pressure.
- Viral infections of neurons . They can be caused by parasites, bacteria, fungi, viruses and prions.
- Asthenia . A symptom can even be a sudden change in character, neurosis or chronic fatigue.
- Mental retardation in children (mental retardation)
Composition of cortexin
Cortexin consists of one main substance “cortexin” and one auxiliary glycine. Nothing more is known about the components of the drug, because it is impossible to disassemble the components of cortexin, even at the molecular level.
The drug is bottled in 5 mm bottles, 10 pieces per package. The price for one pack varies from 780 to 1200 rubles .
Contraindications
- Pregnancy.
- Personal intolerance to the components of the drug.
There are no side effects observed, even with an overdose.
Before buying the drug, consult your doctor; it is not recommended to use it without a prescription.
The important thing is that Cortexin is banned abroad. Scientists have found that the drug contains animal cells, which after a short use aggravate the situation.
Drug interactions
Taking into account the pharmacological profile of the drug Cerebrolysin, special attention should be paid to possible additive effects when co-administered with antidepressants or MAO inhibitors. In such cases, it is recommended to reduce the dose of the antidepressant. The use of Cerebrolysin in high doses (30-40 ml) in combination with MAO inhibitors in high doses can cause an increase in blood pressure.
Cerebrolysin and balanced solutions of amino acids should not be mixed in the same solution for infusion.
Cerebrolysin is incompatible with solutions containing lipids and with solutions that change the pH of the medium (5.0-8.0).
special instructions
If injections are performed too quickly, a feeling of heat, sweating, and dizziness may occur. Therefore, the drug should be administered slowly.
The compatibility of the drug has been tested and confirmed (within 24 hours at room temperature and light) with the following standard solutions for infusion: 0.9% sodium chloride solution, Ringer's solution, 5% dextrose (glucose) solution.
The simultaneous use of Cerebrolysin with vitamins and drugs that improve cardiac circulation is allowed, but these drugs should not be mixed in the same syringe with Cerebrolysin.
Only clear Cerebrolysin solution should be used and only once.
Impact on the ability to drive vehicles and operate machinery
Clinical studies have shown that Cerebrolysin does not affect the ability to drive vehicles and use machinery.
Instructions for use and drug compatibility
Cerebrolysin in dosages of 5 to 10 ml is administered by intravenous injection, and with a dosage of 10 to 50 ml, it is administered by drip, diluting the drug in 100-200 ml of 0.9% sodium chlorine solution.
The course of treatment with medication is from 10 days to 1 month. The duration of therapy, drug administration regimen and dosage are prescribed by the attending doctor depending on the pathology, the complexity of its course and based on the indicators of a diagnostic study of the disease:
neurodegenerative pathologies of brain cells – 5-30 ml. The course of treatment is up to 30 days;- ischemic stroke - 10-50 ml, administered by drip for the first 5 days, then the dosage can be halved and therapy should be carried out for 2 weeks;
- brain injuries and consequences of surgery – 10-50 ml;
- perinatal lesions of the central nervous system in children from the moment of birth - 1-2 ml using intravenous administration. The course of therapy depends on the degree of damage and can last at least a month.
The effectiveness of treatment with Cerebrolysin depends on repeated therapeutic courses, which should be the same in time. The time interval between courses of therapy should be no shorter than the time of the drug course.
Cortexin can be administered intramuscularly, intravenously or by drip. If the administration is carried out using an injection, then the contents of the ampoule are diluted in 1-2 ml of injection solution, and if using a dropper - in 100-200 ml of 0.9% sodium chloride. Treatment regimen and dosage for adult patients and children:
For adults, the medication is administered at a dose of 10 mg for 10-15 days;- during the perinatal period, the child is administered 0.50 mg per kilogram of the infant’s weight. Duration of treatment – 10 days;
- children weighing 20 kg and above - 10 mg per day for 10-14 days; a second course of therapeutic treatment can be carried out after 3 months or six months.
For ischemic stroke, Cortexin and Cerebrolysin are administered simultaneously at a dosage of 10 mg twice a day for 10 days, after which the 10-day course must be repeated if necessary.
Both medications have the same mechanism of action and are compatible for complex treatment, but these medications cannot be mixed in the same syringe. In pediatrics, Cerebrolysin is not advisable to use together with Cortexin, as this can lead to polypharmacy. When, after a course of treatment with Cerebrolysin, the doctor prescribes a second course of therapeutic treatment with nootropics, then it is effective to inject the child with Cortexin.
The simultaneous use of Cortexin with Cerebrolysin in the treatment of cardiac pathologies leads to the restoration of cardiac parameters. For ischemic stroke, these two drugs act equally on the brain, reducing the risk of death.
Release form, composition and packaging
Injection
yellowish-brown, transparent.
| 1 ml | |
| Cerebrolysin concentrate (a complex of peptides* obtained from pig brain) | 215.2 mg |
* molecular weight not more than 10,000 daltons.
Excipients:
sodium hydroxide, water d/i.
- 1 ml - brown glass ampoules (10) - contour cell packaging (1) - cardboard packs.
- 2 ml - brown glass ampoules (10) - contour cell packaging (1) - cardboard packs.
- 5 ml - brown glass ampoules (5) - contour cell packaging (1) - cardboard packs.
- 10 ml - brown glass ampoules (5) - contour cell packaging (1) - cardboard packs.
- 20 ml - brown glass ampoules (5) - contour cell packaging (1) - cardboard packs.
- 30 ml - brown glass bottles (1) - cardboard packs.
- 30 ml - brown glass bottles (5) - cardboard packs.
"Cerebrolysin"
The drug is known among the elderly generation. Mainly used as prescribed by a doctor and sold by prescription. Available in the form of a yellowish injection solution. The price is more than 1000 rubles for a package of 10 ampoules. Not every pensioner can afford such a cost, so there are alternative medications, but they do not work as well or quickly.
Indications for use
- Alzheimer's . This disease occurs in 90% of older people when the cerebral cortex is gradually destroyed. Symptoms may include deterioration of short-term memory in the first stage, confusion in the names of relatives and other things in the second stage. At the last stage (severe dementia), a person may not be able to find his way home at all.
- Ischemic stroke . Poor circulation with damage to brain tissue. As a result, blood stops flowing to certain parts of the brain. A person may suddenly lose consciousness, experience vomiting, nausea, disruption of certain body functions, as well as loss of sensitivity.
- Mechanical damage to the brain or spinal cord . The reasons for this may be a strong blow, a concussion, or a neuron rupture. It is worth noting that in this case treatment is not always necessary.
- ZPR in children . It is manifested by speech delay, poor coordination of the child, severe delayed development compared to generally accepted norms.
- Chronic depression . Keep in mind that even simple depression can develop into chronic depression in a short period of time.